All Photos(3)
About This Item
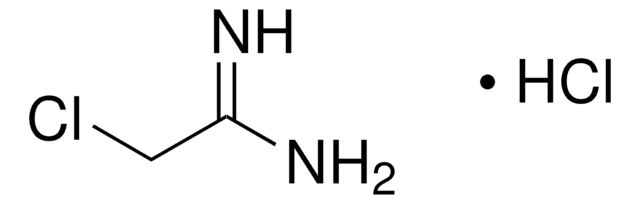
Linear Formula:
CH3C(=NH)NH2 · HCl
CAS Number:
Molecular Weight:
94.54
Beilstein:
3591762
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
mp
165-170 °C (lit.)
SMILES string
Cl[H].CC(N)=N
InChI
1S/C2H6N2.ClH/c1-2(3)4;/h1H3,(H3,3,4);1H
InChI key
WCQOBLXWLRDEQA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Acetamidine hydrochloride is an amidine salt and its conversion to 2,4,6-trimethyl-sym-triazine has been studied.
Application
Acetamidine hydrochloride was used in the preparation of decarboxyectoine. It was also used in the synthesis of ethyl 4-(4-hydroxyphenyl)methylidene-2-methyl-5-oxo-1-imidazolacetate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of the sym-Triazine System. I. Trimerization and Cotrimerization of Amidines.
Schaefer FC, et al.
Journal of the American Chemical Society, 81(6), 1466-1470 (1959)
B Turano et al.
Biophysical journal, 63(1), 152-161 (1992-07-01)
Empirical energy function calculations were used to evaluate the effects of minimization on the structure of a gramicidin A channel and to analyze the energies of interaction between three cations (guanidinium, acetamidinium, formamidinium) and the channel as a function of
Antonia Patruno et al.
Biochimica et biophysica acta, 1820(12), 2095-2104 (2012-09-07)
Previous reports suggest that NO may contribute to the pathophysiology of septic shock. Recently, we have synthesized and characterized a series of benzyl- and dibenzyl derivative of N-(3-aminobenzyl)acetamidine, a potent and selective inhibitor of iNOS, in vitro assay. We evaluated
G Hemsley et al.
Biophysical journal, 59(4), 901-907 (1991-04-01)
Guanidinium and acetamidinium, when added to the bathing solution in concentrations of approximately 0.1M, cause brief blocks in the single channel potassium currents from channels formed in planar lipid bilayers by gramicidin A. Single channel lifetimes are not affected indicating
M M Ng et al.
Biochemistry, 33(40), 12119-12126 (1994-10-11)
Isoguanosine has been incorporated into a 34-mer hammerhead ribozyme by the solid-phase phosphoramidite method, using an acetamidine base protecting group. The activity of the hammerhead ribozyme when singly mutated to isoguanosine at the adenosine positions 6, 9, and 13 was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service