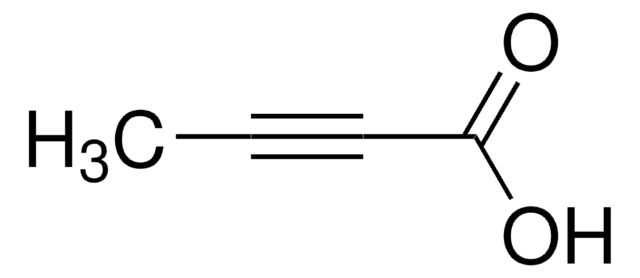All Photos(1)
About This Item
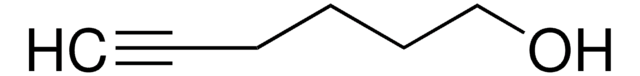
Linear Formula:
HC≡C(CH2)4COOH
CAS Number:
Molecular Weight:
126.15
Beilstein:
1747024
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
90%
refractive index
n20/D 1.451 (lit.)
bp
93-94 °C/1 mmHg (lit.)
density
0.997 g/mL at 25 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)CCCCC#C
InChI
1S/C7H10O2/c1-2-3-4-5-6-7(8)9/h1H,3-6H2,(H,8,9)
InChI key
OFCPMJGTZUVUSM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6-Heptynoic acid is an alkynoic acid with an acetylene bond. It undergoes condensation with various pyrroles to afford optical diverse fluorescent dyes with a terminal alkyne.
Application
6-Heptynoic acid may be used for the following syntheses:
- alkyne functionalized Boradiazaindacenes (BODIPY)dyes
- natural products epothilone B and D
- hymenialdisine (HMD) and aldisine (AD) affinity resins
- alkynyl esters
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lauren Ray et al.
Nature communications, 7, 13609-13609 (2016-12-22)
Type I modular polyketide synthases assemble diverse bioactive natural products. Such multienzymes typically use malonyl and methylmalonyl-CoA building blocks for polyketide chain assembly. However, in several cases more exotic alkylmalonyl-CoA extender units are also known to be incorporated. In all
Martijn Verdoes et al.
Bioorganic & medicinal chemistry letters, 17(22), 6169-6171 (2007-09-25)
The synthesis of three acetylene functionalized BODIPY dyes is described. These dyes are used to fluorescently modify an azido functionalized epoxomicin analogue employing the Huisgen 1,3-dipolar cycloaddition, resulting in a panel of fluorescent epoxomicin derived proteasome probes.
R E Taylor et al.
Organic letters, 3(14), 2221-2224 (2001-07-07)
[reaction: see text] A highly convergent total synthesis of the natural products epothilone B and D is described. The route is highlighted by efficient generation of a C12-C13 trisubstituted olefin which exploits a sequential Nozaki-Hiyama-Kishi coupling and a stereoselective thionyl
Yongqin Wan et al.
Chemistry & biology, 11(2), 247-259 (2004-05-05)
Hymenialdisine (HMD) is a sponge-derived natural product kinase inhibitor with nanomolar activity against CDKs, Mek1, GSK3beta, and CK1 and micromolar activity against Chk1. In order to explore the broader application of the pyrrolo[2,3-c]azepine skeleton of HMD as a general kinase
A novel catalyst with a cuboidal PdMo3S4 core for the cyclization of alkynoic acids to enol lactones.
Wakabayashi T, et al.
Angewandte Chemie (International Edition in English), 35(18), 2123-2124 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service