232211
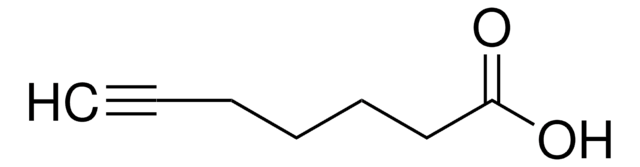
4-Pentynoic acid
95%
Synonym(s):
Propargylacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH≡CCH2CH2COOH
CAS Number:
Molecular Weight:
98.10
Beilstein:
1742047
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
bp
110 °C/30 mmHg (lit.)
mp
54-57 °C (lit.)
functional group
carboxylic acid
storage temp.
2-8°C
SMILES string
OC(=O)CCC#C
InChI
1S/C5H6O2/c1-2-3-4-5(6)7/h1H,3-4H2,(H,6,7)
InChI key
MLBYLEUJXUBIJJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Pentynoic acid undergoes copper-catalyzed intramolecular cyclizations to form enol lactones. It also reacts with 1-bromo-1-alkynes in the presence of a Pd catalyst to yield biologically active ynenol lactones.
Application
4-Pentynoic acid was used:
- as building block for the synthesis of library of eight sequence-defined model oligomers
- in one-pot synthesis of the complex polycyclic heterocycles benzo[4,5]imidazo[1,2-c]pyrrolo[1,2-a]quinazolinone derivatives
- in the synthesis of various allenenols lactones [5(E)-(2-allenylidene)-tetrahydro-2-furanones]
- in the synthesis of a cyctotoxic macrolide by ring-closing metathesis of a bis acetylene
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Palladium-catalyzed synthesis of new unsaturated exo-enol lactones with potential biological activity.
Bouyssi D, et al.
Tetrahedron Letters, 34(19), 3129-3130 (1993)
Xun Ji et al.
The Journal of organic chemistry, 78(9), 4312-4318 (2013-04-06)
An efficient and facile Au(I)/Ag(I)-catalyzed cascade method has been developed for one-pot synthesis of the complex polycyclic heterocycles benzo[4,5]imidazo[1,2-c]pyrrolo[1,2-a]quinazolinone derivatives through treatment of the substituted 2-(1H-benzo[d]imidazol-2-yl)anilines with 4-pentynoic acid or 5-hexynoic acid. The strategy features a Au(I)/Ag(I)-catalyzed one-pot cascade process
Thomas L Mindt et al.
The Journal of organic chemistry, 72(26), 10247-10250 (2007-11-30)
Alkynoic acids, in particular, 4-pentynoic acid derivatives, undergo intramolecular cyclizations to enol lactones under reaction conditions typically applied for the Cu(I)-catalyzed cycloaddition of terminal alkynes and azides (click chemistry). Starting from appropriate alkynoic acid derivatives, either enol lactones or 1,2,3-triazole
Tetrahedron Letters, 33, 2811-2811 (1992)
Thanh Tam Trinh et al.
Macromolecular rapid communications, 35(2), 141-145 (2013-12-18)
A library of eight sequence-defined model oligomers, whose sequence is based on a (0,1) binary code, is prepared through chemoselective repeating cycles of amidification and copper-assisted alkyne-azide cycloaddition reactions from a non-modified Wang resin. This library is constructed from two
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service