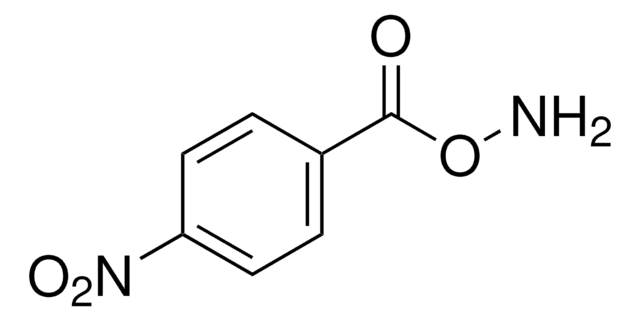
B22984
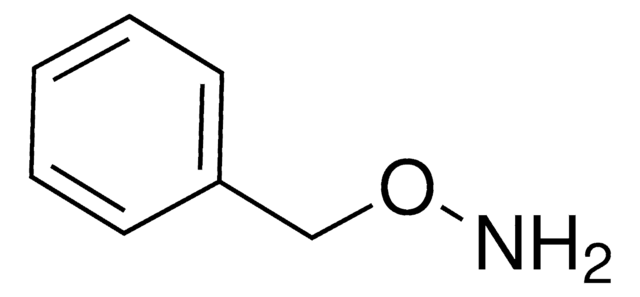
O-Benzylhydroxylamine hydrochloride
99%
Sinónimos:
Benzyloxyamine hydrochloride
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6H5CH2ONH2 · HCl
Número de CAS:
Peso molecular:
159.61
Beilstein/REAXYS Number:
3687991
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
99%
form
crystals
mp
238 °C (subl.) (lit.)
SMILES string
Cl.NOCc1ccccc1
InChI
1S/C7H9NO.ClH/c8-9-6-7-4-2-1-3-5-7;/h1-5H,6,8H2;1H
InChI key
HYDZPXNVHXJHBG-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Effective reagent used to prepare α-hydroxybenzylamines from α-hydroxyketones.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Tetrahedron Letters, 32, 711-711 (1991)
D F Magin
Journal of chromatography, 202(2), 255-261 (1980-12-19)
A qualitative and semi-quantitative method was established for the investigation of low-molecular-weight volatile carbonyl compounds in cigarette whole smoke. The carbonyls were trapped on a silica gel "column" and eluted with water. The aqueous solution was then treated with benzyloxyamine
G Cardillo et al.
Organic letters, 3(8), 1165-1167 (2001-05-12)
[reaction: see text]. The 1,4-addition of O-benzylhydroxylamine to alpha,beta-unsaturated imide 1 in the presence of BF3.Et2O proceeds with the preferential attack of the nucleophile on the Cbeta-re face. To explain this unexpected reactivity 1H, 13C, and 11B NMR investigations have
Yin Luo et al.
ChemMedChem, 7(9), 1587-1593 (2012-07-20)
Forty-three oxime derivatives were synthesized by allowing O-benzylhydroxylamines to react with primary benzaldehydes or salicylaldehydes; these products were gauged as potential inhibitors of β-ketoacyl-(acyl-carrier-protein) synthase III (FabH). Among the 43 compounds, 38 are reported herein for the first time. These
Han Fu et al.
Journal of combinatorial chemistry, 9(5), 804-810 (2007-08-11)
A highly regioselective and traceless solid-phase route to 1,7,8-trisubstituted purines has been developed. This methodology could be extended to the preparation of 8-azapurines and [i]-condensed purines. A representative set of 17 purines, azapurines, and [i]-condensed purines was synthesized. This paper
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico