671576
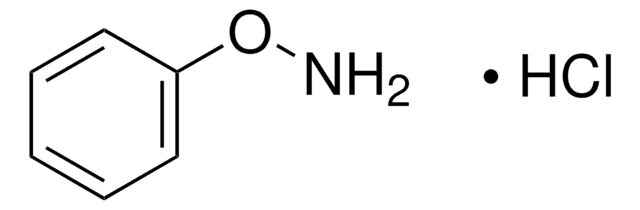
N-Phenylhydroxylamine
≥95.0%
Sinónimos:
N-Hydroxybenzenamine
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C6H7NO
Número de CAS:
Peso molecular:
109.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
solid
assay:
≥95.0%
Productos recomendados
Quality Level
assay
≥95.0%
form
solid
mp
80-84 °C
storage temp.
−20°C
SMILES string
ONc1ccccc1
InChI
1S/C6H7NO/c8-7-6-4-2-1-3-5-6/h1-5,7-8H
InChI key
CKRZKMFTZCFYGB-UHFFFAOYSA-N
Categorías relacionadas
Application
N-Phenylhydroxylamine can be used as a starting material for the synthesis of:
- 2-alkylindoles by treating with aliphatic terminal alkynes using gold catalyst via sequential 3,3-rearrangements and cyclodehydrations.
- Isoxazolidines by reacting with aldehydes and α, β-unsaturated aldehydes via a three-component one-pot catalytic reaction.
- Tetrahydro-1,2-oxazines by treating with an aldehyde and cyclopropane via homo 3+2 dipolar cycloaddition reaction.
signalword
Danger
hcodes
pcodes
Hazard Classifications
Acute Tox. 3 Oral
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Copper-catalyzed amination of alkenes and ketones by phenylhydroxylamine.
Ho C-M and Lau T-C
New. J. Chem., 24(11), 859-863 (2000)
Au-catalyzed synthesis of 2-alkylindoles from N-arylhydroxylamines and terminal alkynes
Wang Y, et al.
Chemical Communications (Cambridge, England), 47(27), 7815-7817 (2011)
Y Endo et al.
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 114(8), 565-576 (1994-08-01)
The acid-catalyzed reaction of N-acyl- and N-sulfonylhydroxylamines with benzene proceeded smoothly to give C-C products; 2- and 4-hydroxybiphenyls. The reaction and the thermolysis of N-aryloxypyridinium salts involve common intermediates. The results of product analysis, the orientation of the reaction, effects
T P Bradshaw et al.
Free radical biology & medicine, 18(2), 279-285 (1995-02-01)
Previous studies have shown that incubation of rat red blood cells in vitro with phenylhydroxylamine (50-300 microM) induces rapid splenic sequestration of the red cells on reintroduction to isologous rats. EPR and the spin trapping agent, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), were utilized
Vance G Nielsen et al.
Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis, 22(8), 657-661 (2011-08-09)
Cigarette smoking is associated with plasmatic hypercoagulability, and carbon monoxide has been demonstrated to enhance coagulation by binding to a fibrinogen-bound heme. Our objective was to design and test a redox-based method to detect carboxyhemefibrinogen. Normal, pooled, citrated plasma was
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico