A1075
Amyloid β Protein Fragment 1-40
≥90% (HPLC), powder
Synonym(s):
Aβ40
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C194H295N53O58S
CAS Number:
Molecular Weight:
4329.80
MDL number:
UNSPSC Code:
12352202
NACRES:
NA.32
Recommended Products
Quality Level
Assay
≥90% (HPLC)
form
powder
color
white
solubility
1% acetic acid: 1 mg/mL
saline: insoluble
UniProt accession no.
storage temp.
−20°C
Gene Information
human ... APP(351)
Looking for similar products? Visit Product Comparison Guide
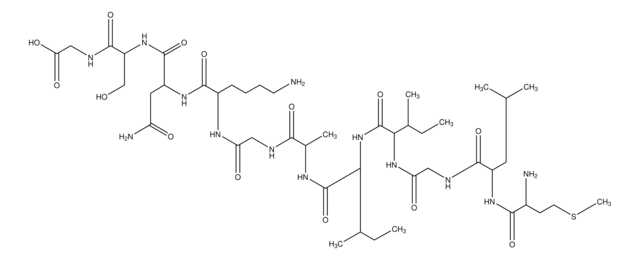
Amino Acid Sequence
Asp-Ala-Glu-Phe-Arg-His-Asp-Ser-Gly-Tyr-Glu-Val-His-His-Gln-Lys-Leu-Val-Phe-Phe-Ala-Glu-Asp-Val-Gly-Ser-Asn-Lys-Gly-Ala-Ile-Ile-Gly-Leu-Met-Val-Gly-Gly-Val-Val
General description
Amyloid β Protein Fragment 1-40 (Aβ40) is derived from the amyloid-β protein (Aβ), which is mapped to human chromosome 21q21.3. Aβ40 is predominantly present in the vascular amyloid deposits. Aβ40 comprises of C-terminal membrane insertion domain. It shows structural transition from random coil to a α-helical structure in a water-micelle medium.
Application
Amyloid β Protein Fragment 1-40 has been used:
- in the temperature based conformational studies using Fourier transform infrared/differential scanning calorimetry (FT-IR/DSC) studies
- as a reference standard in sandwich-type enzyme immunoassay for quantifying amyloid A4 protein in cerebrospinal fluid of patients with head trauma
- as a component of embryonic stem cell medium to inhibit amyloid deposition in fibroblasts
Biochem/physiol Actions
Amyloid β Protein Fragment 1-40 (Aβ40) forms cation based ion channels.
Amyloid β-protein is neurotrophic and neurotoxic in vivo and in vitro in human and rat neuronal cell cultures. β-Amyloid peptides (amino acids 1-42 and 1-43) are the major constituents of senile plaques and neurofibrillary tangles that occur in the hippocampus, neocortex, and amygdala of patients with Alzheimer′s disease.
Reconstitution
For maximal biological activity, dilute the stock in calcium-free PBS to 1 mg/ml and incubate at 37 °C for 4 days.
Other Notes
Lyophilized from 0.1% TFA in H2O
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H Funato et al.
The American journal of pathology, 152(4), 983-992 (1998-04-18)
Amyloid beta-protein (Abeta) is the major component of senile plaques that emerge in the cortex during aging and appear most abundantly in Alzheimer's disease. In the course of our immunocytochemical study on a large number of autopsy cases, we noticed
D R Howlett et al.
Neurodegeneration : a journal for neurodegenerative disorders, neuroprotection, and neuroregeneration, 4(1), 23-32 (1995-03-01)
The behaviour of synthetic batches of beta-amyloid (beta A) 1-40 peptide in solution has been studied. The effects of beta A1-40 on a PC12 cell toxicity assay was dependent upon the time of preincubation of an aqueous solution of the
M Citron et al.
Proceedings of the National Academy of Sciences of the United States of America, 93(23), 13170-13175 (1996-11-12)
Cerebral deposition of the amyloid beta protein (A beta) is an early and invariant feature of Alzheimer disease (AD). Whereas the 40-amino acid form of A beta (A beta 40) accounts for approximately 90% of all A beta normally released
Amyloid beta protein 1-40 and 1-42 levels in matched cerebrospinal fluid and plasma from patients with Alzheimer disease
Mehta PD, et al.
Neuroscience Letters, 304(1-2), 102-106 (2001)
K Ueda et al.
Brain research, 639(2), 240-244 (1994-03-14)
The neurotoxic effects of soluble and aggregated synthetic amyloid beta protein (A beta P) have been investigated in rat primary cultures. Freshly solubilized beta(1-40) was neurotoxic not to immature, but to mature hippocampal neurons. On the other hand, aggregated beta(1-40)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![[Gln22]-Amyloid β 1-40 human ≥95% (HPLC)](/deepweb/assets/sigmaaldrich/product/images/707/874/59f84b84-17c2-494f-b2ae-4ca860b83976/640/59f84b84-17c2-494f-b2ae-4ca860b83976.jpg)