Differentiation of Primary Human Osteoblasts
Osteoblasts (HOB) are specialized fibroblast cells that secrete and mineralize the bone matrix. They develop from mesenchymal precursors. The mineralized extracellular matrix is mainly composed of type I collagen and smaller but significant amounts of osteocalcin (OC), matrix gla protein, osteopontin (OPN), bone sialoprotein (BSP), BMPs, TGFβ, and the inorganic mineral hydroxylapatite. Osteoblast differentiation can be characterized in three stages: (a) cell proliferation, (b) matrix maturation, and (c) matrix mineralization.1 In vitro, matrix maturation and mineralization are usually enhanced by growing the cells to complete confluency and by adding specific osteogenic factors.2 During proliferation, several extracellular matrix proteins (procollagen I, TGFβ, and fibronectin) can be detected. The matrix maturation phase is characterized by maximal expression of alkaline phosphatase (AP). Finally, at the beginning of matrix mineralization, genes for proteins such as OC, BSP, and OPN are expressed and once mineralization is completed, calcium deposition can be visualized using adequate staining methods. Analysis of bone cellspecific markers like AP, OC, and collagen type I or detection of fuctional mineralization is frequently used to characterize osteoblasts in vitro.2 The mineralization process of osteoblasts in in vitro culture has also been used as a model for testing the effects of drug treatments and mechanical loading on bone cell differentiation and bone formation.3, 4 PromoCell offers primary human osteoblasts isolated from adult tissue of individual donors (C12720, C12760) along with optimized growth (C27001) and mineralization (C27020) media to ensure consistent cell culture performance.

Figure 1.Overview of osteoblast bone formation and mineralization.
Osteoblast Differentiation Protocol
- Seed osteoblasts (HOB) on collagen precoated culture plates. Plate 3x104 HOB per well on a collagen, type 1 (C3867) coated 24well tissue culture plate. Work in duplicate. Use HOB Growth Medium (C-27001) for one well as a negative control and Osteoblast Mineralization Medium (C-27020) for the other well.
- Differentiation culture of induced osteoblasts. Incubate the cells for 17–21 days. Change the medium every third day. Be careful not to disturb the cell monolayer.
Detection of Calcium Deposits (Mineralization)
- Wash the cells. Remove the cells from the incubator and carefully aspirate the medium. Carefully wash the cells with Dulbecco’s PBS, w/o Ca++/ Mg++ (D8537).
- Fix the cells. Carefully aspirate the PBS and add enough Saccomanno Fixation Solution to cover the cellular monolayer. After at least 60 min gently aspirate the fixation solution and wash the cells with distilled water.
- Stain the cells. Immediately before use, pass the required amount of Alizarin Red S staining solution (TMS008) through a 0.22 μM Millex PES filter. Carefully aspirate the distilled water and add enough filtered Alizarin Red S staining solution to cover the cellular monolayer. Incubate at room temperature in the dark for 45 min.
- Wash the cells. Carefully aspirate the Alizarin Red S staining solution and wash the cell monolayer four times with 1 ml distilled water. Carefully aspirate the distilled water and add PBS.
- Analyze the sample immediately, as the dye may bleed upon prolonged storage without embedding. Undifferentiated HOB (without extracellular calcium deposits) are slightly reddish, whereas mineralized osteoblasts (with extracellular calcium deposits) are bright orangered.
Detection of Alkaline Phosphatase
- Prepare solutions and reagents. Dissolve one BCIP/NBT tablet (B5655) in 10 ml distilled water to prepare the substrate solution. Store in the dark and use within 2 hours. Add 0.05% Tween 20 (P2287) to PBS, w/o Ca++/ Mg++ (D8537) to prepare the washing buffer.
- Wash the cells. Remove the cells from the incubator and carefully aspirate the medium. Carefully wash the cells with PBS.
- Fix the cells. Carefully aspirate the PBS and add enough Saccomanno Fixation Solution to cover the cellular monolayer. After 60 90 seconds gently aspirate the fixation solution and wash the cells with washing buffer. Note: Longer fixation will lead to irreversible inactivation of AP.
- Stain the cells. Carefully aspirate the washing buffer and add enough BCIP/NBT substrate solution to cover the cellular monolayer. Incubate at room temperature in the dark for 510 min. Check staining progress every 23 min.
- Wash the cells. Carefully aspirate the substrate solution and wash the cell monolayer with washing buffer. Carefully aspirate the washing buffer and add PBS.
- Analyze the cells. Evaluate staining results.

Figure 2. Microscopic appearance of HOB after mineralization in vitro. A) Mineralized osteoblasts in Osteoblast Mineralization Medium show vast extracellular calcium deposits, stained in bright orange-red. Right: The negative control in HOB Growth Medium is slightly reddish.
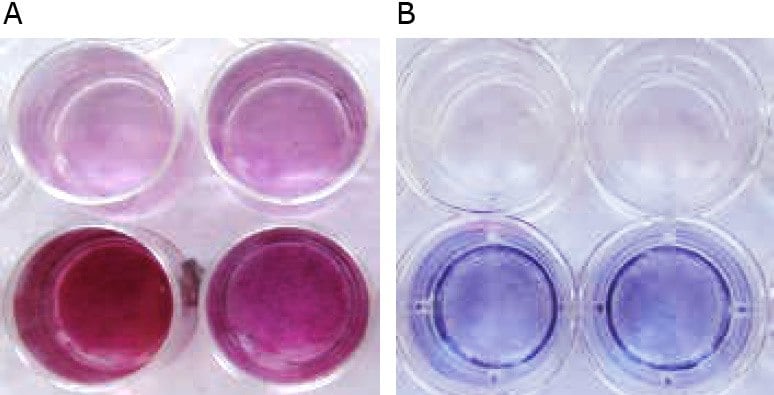
Figure 3. Macroscopic appearance of HOB after mineralization in vitro. A) The negative control in Osteoblast Growth Medium (upper row) is slightly reddish, whereas the mineralized osteoblasts in Osteoblast Mineralization Medium show vast extracellular calcium deposits, stained in bright orange-red (lower row). B) HUVEC (AP negative, upper row) are colorless or faintly bluish, whereas osteoblasts (AP positive, lower row) are dark blue-violet. The higher the AP activity, the more intense the color.
References
To continue reading please sign in or create an account.
Don't Have An Account?