H6660
HIV Protease Substrate 1
≥95% (HPLC), powder
Synonym(s):
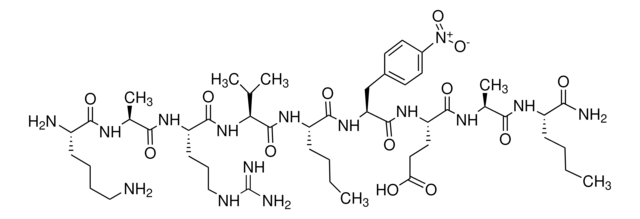
Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
product name
HIV Protease Substrate 1,
assay
≥95% (HPLC)
Quality Level
form
powder
mol wt
2016
solubility
DMSO: soluble
storage temp.
−20°C
Application
HIV Protease Substrate 1 has been used:
- to study protein structure-function relationships and in the fluorescent assay
- to evaluate protease inhibitors and for in vitro detection of the HIV-1 protease activity
- and in protease kinetics to study its enzymatic degradation rates
Biochem/physiol Actions
HIV Protease Substrate 1 is a synthetic peptide sequence that contains the cleavage site (Tyr-Pro) for the human immunodeficiency virus (HIV) Protease. It has two covalently modified amino acids for the detection of cleavage. One modification is the addition of the fluorophore 5-(2-aminoethylamino)-1-naphthalene sulfonate (EDANS) to the glutamic acid residue, while the other one includes the addition of the acceptor chromophore 4c-dimethylaminoazobenzene-4-carboxylate (DABCYL) to the lysine residue. The modified amino acids are on opposite sides of the cleavage site. Spatial orientation and overlap of the DABCYL absorbance with the EDANS emission permit resonance energy transfer between the two moieties. However, when the peptide is cleaved by the HIV protease, the DABCYL group is no longer proximal to the fluorophore.
Substrate for endopeptidases
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nancy Cheng et al.
Journal of laboratory automation, 19(3), 297-303 (2013-12-07)
Current antiretroviral treatments target multiple pathways important for human immunodeficiency virus (HIV) multiplication, including viral entry, synthesis and integration of the DNA provirus, and the processing of viral polyprotein precursors. However, HIV is becoming increasingly resistant to these "combination therapies."
Sagheer A Onaizi et al.
Langmuir : the ACS journal of surfaces and colloids, 23(11), 6336-6341 (2007-04-24)
We present the first study of the directed disassembly of a protein network at the air-water interface by the synergistic action of a surfactant and an enzyme. We seek to understand the fundamentals of protein network disassembly by using rubisco
Rok Gaber et al.
Sensors (Basel, Switzerland), 13(12), 16330-16346 (2013-11-30)
To effectively fight against the human immunodeficiency virus infection/ acquired immunodeficiency syndrome (HIV/AIDS) epidemic, ongoing development of novel HIV protease inhibitors is required. Inexpensive high-throughput screening assays are needed to quickly scan large sets of chemicals for potential inhibitors. We
Jason Segura et al.
PloS one, 18(2), e0281087-e0281087 (2023-02-14)
HIV infection remains incurable to date and there are no compounds targeted at the viral release. We show here HIV viral release is not spontaneous, rather requires caspases activation and shedding of its adhesion receptor, CD62L. Blocking the caspases activation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service