912794
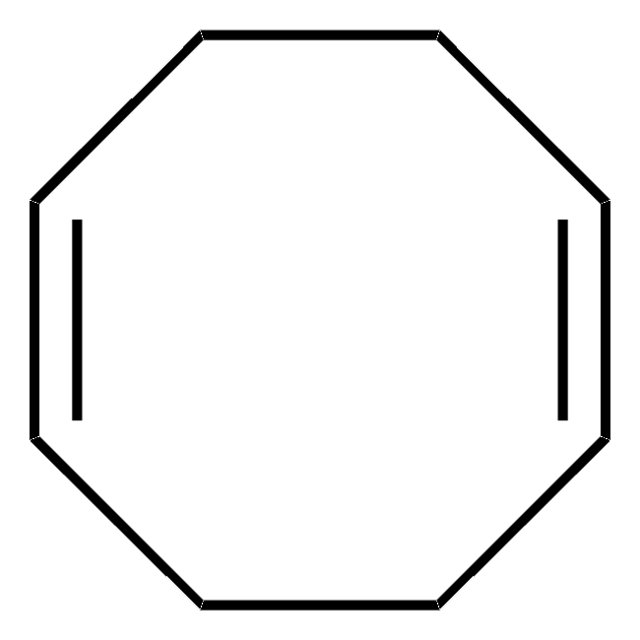
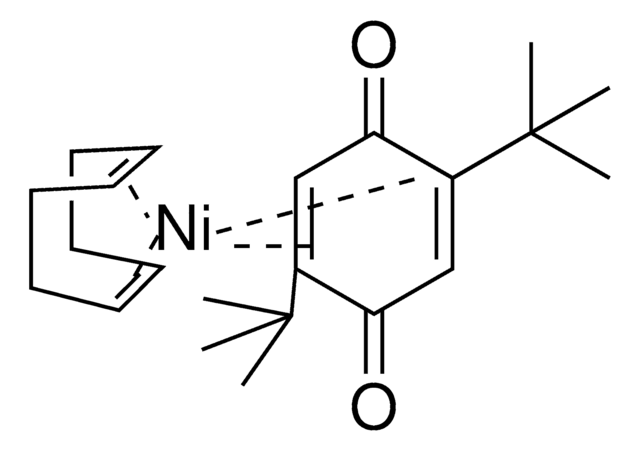
Ni(COD)(DQ)
≥95%
Synonym(s):
Bis(1,5-cyclooctadiene)(duroquinone) nickel(0)
About This Item
Recommended Products
Quality Level
assay
≥95%
form
powder
reaction suitability
reagent type: catalyst
reaction type: Cross Couplings
parameter
temperature stable
mp
227 °C (decomposition)
Application
related product
signalword
Danger
hcodes
Hazard Classifications
Carc. 2 - Skin Sens. 1 - STOT RE 1
target_organs
Lungs
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service