All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H16OSi
CAS Number:
Molecular Weight:
168.31
Beilstein/REAXYS Number:
2079140
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
95%
form
liquid
refractive index
n20/D 1.462 (lit.)
bp
65 °C/7 mmHg (lit.)
density
0.899 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
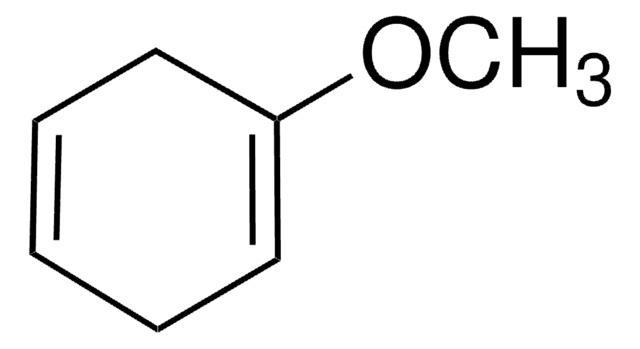
C[Si](C)(C)OC1=CCCC=C1
InChI
1S/C9H16OSi/c1-11(2,3)10-9-7-5-4-6-8-9/h5,7-8H,4,6H2,1-3H3
InChI key
WPIRVUXAMPRMAY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-(Trimethylsiloxy)-1,3-cyclohexadiene ((cyclohexa-1,5-dien-1-yloxy)trimethylsilane) is silyl enol ether of cyclohexenone. The Diels–Alder reactions with dienophiles α-acetoxyacrylonitrile, acrylonitrile and α-chloroacrylonitrile has been studied.
Application
2-(Trimethylsiloxy)-1,3-cyclohexadiene may be used as a diene in the synthesis of isoquinuclidinone 2-aza[2.2.2]octa-3,5-dione by reacting with p-toluenesulfonyl cyanide.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Safety Assessment of Diels-Alder Reactions with Highly Reactive Acrylic Monomers.
Abele S, et al.
Organic Process Research & Development, 16(12), 2015-2020 (2012)
Design and Scale-Up of Diels-Alder Reactions for the Practical Synthesis of 5-Phenylbicyclo [2.2. 2] oct-5-en-2-one.
Funel JA, et al.
Organic Process Research & Development, 15(6), 1420-1427 (2011)
High-Temperature Diels-Alder Reactions: Transfer from Batch to Continuous Mode.
Abele S, et al.
Organic Process Research & Development, 16(5), 1114-1120 (2011)
Cynthia K McClure et al.
The Journal of organic chemistry, 68(21), 8256-8257 (2003-10-11)
The hetero-Diels-Alder reaction of an electron-deficient nitrile, p-toluenesulfonyl cyanide, with the silyl enol ether of cyclohexenone produced a hydrolytically sensitive [4 + 2] adduct in good yield. Use of Mander's reagent, ethyl cyanoformate, with the same diene, produced an unstable
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service