156361
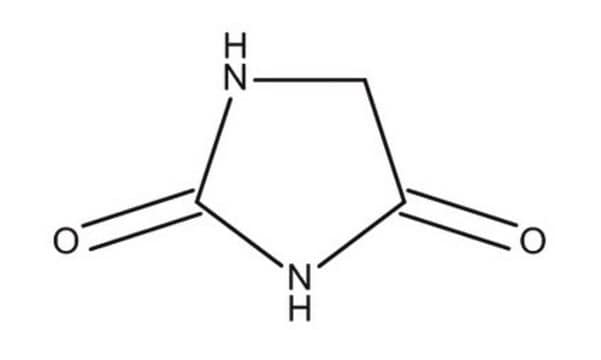
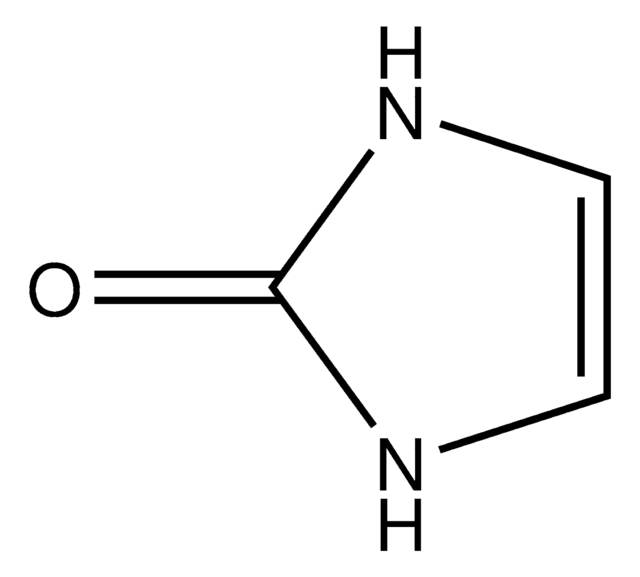
Hydantoin
98%
Synonym(s):
2,4-Imidazolidinedione, Glycolylurea
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H4N2O2
CAS Number:
Molecular Weight:
100.08
Beilstein/REAXYS Number:
110598
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
powder
assay:
98%
Recommended Products
Quality Level
assay
98%
form
powder
mp
218-220 °C (lit.)
SMILES string
O=C1CNC(=O)N1
InChI
1S/C3H4N2O2/c6-2-1-4-3(7)5-2/h1H2,(H2,4,5,6,7)
InChI key
WJRBRSLFGCUECM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for synthesis of:
N-benzyl aplysinopsin analogs as anticancer agents
D-glutamic acid based inhibitors
Antidiabetic chromonyl-2,4-thiazolidinediones
GSK-3β inhibitors with brain permeability
Thiazolidinedione derivatives as 15-PGDH inhibitors
Radio-sensitizing agents
N-benzyl aplysinopsin analogs as anticancer agents
D-glutamic acid based inhibitors
Antidiabetic chromonyl-2,4-thiazolidinediones
GSK-3β inhibitors with brain permeability
Thiazolidinedione derivatives as 15-PGDH inhibitors
Radio-sensitizing agents
The product has been used as a substrate (at 40 °C and pH 9.0) to determine the D-hydantoinase activity in adzuki bean extract.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
I Hauksson et al.
The British journal of dermatology, 174(2), 371-379 (2015-10-20)
Formaldehyde is a well-known contact sensitizer. Formaldehyde releasers are widely used preservatives in skincare products. It has been found that formaldehyde at concentrations allowed by the European Cosmetics Directive can cause allergic contact dermatitis. However, we still lack information on
June Izquierdo et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(53), 12431-12438 (2019-07-19)
A bifunctional amine/squaramide catalyst promoted direct aldol addition of an hydantoin surrogate to pyridine 2-carbaldehyde N-oxides to afford adducts bearing two vicinal tertiary/quaternary carbons in high diastereo- and enantioselectivity (d.r. up to >20:1; ee up to 98 %) is reported. Acid
Lixuan Zhan et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 33(1), 1313-1329 (2018-08-28)
Hypoxic preconditioning (HPC) alleviates the selective and delayed neuronal death in the hippocampal CA1 region induced by transient global cerebral ischemia (tGCI). This type of cell death may include different programmed cell death mechanisms, namely, apoptosis and necroptosis. Although apoptotic
Y Thirupathi Reddy et al.
Bioorganic & medicinal chemistry letters, 20(2), 600-602 (2009-12-17)
A series of (Z)-5-((N-benzyl-1H-indol-3-yl)methylene)imidazolidine-2,4-dione (9a-9m) and 5-((N-benzyl-1H-indol-3-yl)methylene)pyrimidine-2,4,6(1H,3H,5H)-trione (10a-10i) derivatives that incorporate a variety of aromatic substituents in both the indole and N-benzyl moieties have been synthesized. These analogs were evaluated for their radiosensitization activity against the HT-29 cell line. Three
Narsimha Reddy Penthala et al.
Bioorganic & medicinal chemistry letters, 21(5), 1411-1413 (2011-02-08)
A series of novel substituted (Z)-5-((1-benzyl-1H-indol-3-yl)methylene)imidazolidin-2,4-diones (3a-f) and (Z)-5-((1-benzyl-1H-indol-3-yl)methylene)-2-iminothiazolidin-4-ones (3g-o) have been synthesized utilizing microwave irradiation. These analogs were evaluated for in vitro cytotoxicity against a panel of 60 human tumor cell lines. Compound 3i exhibits potent growth inhibition against
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service