119725
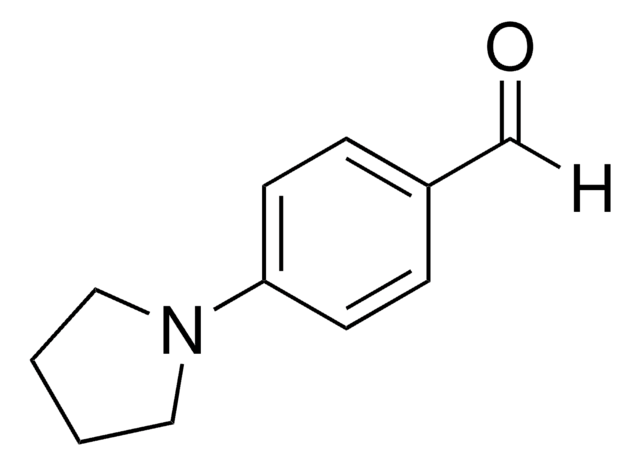
4′-Piperidinoacetophenone
97%
Synonym(s):
1-[4-(1-Piperidinyl)]phenyl]ethanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H17NO
CAS Number:
Molecular Weight:
203.28
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
85-87 °C (lit.)
functional group
ketone
SMILES string
CC(=O)c1ccc(cc1)N2CCCCC2
InChI
1S/C13H17NO/c1-11(15)12-5-7-13(8-6-12)14-9-3-2-4-10-14/h5-8H,2-4,9-10H2,1H3
InChI key
JCMZZYSPSGHBNM-UHFFFAOYSA-N
General description
4′-Piperidinoacetophenone undergoes Claisen-Schmidt condensation with substituted benzaldehydes using NaOH-Al2O3 by microwave irradiation to give chalcones. It has antimycobacterial activity.
Application
4′-Piperidinoacetophenone was used as internal standard in the determination of pethidine, a narcotic analgesic drug in body fluids by gas chromatography-tandem mass spectrometry.
Pharmacological activity:
- Selective antimycobacterial activity
- Bronchodilator compound
- Modulation of flagellar motility in Chlamydomonas
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Akira Ishii et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 792(1), 117-121 (2003-06-28)
We have presented a simple and sensitive method for determining pethidine, a narcotic analgesic drug in body fluids by gas chromatography-tandem mass spectrometry (GC-MS/MS). Pethidine and 4'-piperidinoacetophenone (internal standard) were extracted from body fluids with Bond Elut C(18) columns; the
Microwave enhanced Claisen-Schmidt condensation: A green route to chalcones.
Singh JP, et al.
Indian J. Chem. B, 51(11), 1623-1623 (2012)
L Rajabi et al.
Letters in applied microbiology, 40(3), 212-217 (2005-02-18)
Mycobacteria are a serious cause of infections in humans, with limited treatment options, as no new antibiotics have been developed against mycobacteria since the 1960s. In this study, the antimycobacterial activity of a small library of acetophenone (AP) compounds was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service