Headspace-SPME for Insect Infestation Detection
Abstract
Isopentenols and polysulfides have been reported as potential early biomarkers for the presence of insects (moths and beetles) in rice. The aim of this study was to develop a headspace solid phase microextraction (HS-SPME-GC-MS) method for high-throughput analysis and detection of early volatile biomarkers (prenol, prenal, isopentenol, hexanal, dimethyl disulfide, dimethyl trisulfide, 2-methylfuran, and 2-pentylfuran) in rice as previously used experimentally as biomarkers.1 After examination of 4 commercially available SPME coatings, Carboxen®-PDMS fiber coating was found to be most effective in the extraction and desorption of the volatile components compared to the other fibers. We demonstrated that HS-SPME can be used as a fast and versatile insect monitoring method in integrated pest management (IPM) programs.
Introduction
Stored grains can be infested by a variety of pests that can cause brain damage and affect their quality and nutritional standards. Pest infestation in stored rice is responsible for postharvest losses of 9% in developed countries and even larger worldwide. Typical insect pest control methods that implement chemical insecticides have been gradually replaced by modern stored-product integrated pest management (IPM) programs that represent an eco-friendly and environmentally safe approach to pest control.2 IPM decision-making is based on knowledge of population dynamics and threshold insect density, where appropriate monitoring
tools are of great importance.1,2 A variety of monitoring methods are employed. For instance, pheromone traps are typically used as a monitoring method, in which adult insects are targeted. However, an adult female insect can produce hundreds of eggs before being detected which could delay pest control actions.1,3 Thus, the use of new monitoring methods for early insect detection would be highly beneficial for fine-tuning and improving IPM programs.
All living organisms present in the environment produce a wide range of volatile organic compounds (VOCs) in different stages of their life cycle. Nowadays, VOCs are used as biomarkers, particularly benzoquinones, hydrocarbons, alcohols, furans, and aldehydes are used as insect biomarkers which can be characteristic of a determinate insect species.1-5 Monitoring methods that allow the detection of specific VOCs resulting from the activity of the larvae in the early stages of insect infestations are needed in IPM programs. Thus, detecting the presence of insects at low densities and early stages of development allows us to implement corrective actions and avoid total deterioration of stored grains.1 In this regard, solid phase microextraction (SPME) is a viable alternative as a sample preparation method, as will be shown here. Compared to other preconcentration techniques, SPME is simple, inexpensive, and solvent-free. It is fully automatable, and no thermal desorption unit or modifications to the GC instrument are necessary. Compatible with all GC systems, SPME can be used by practically every laboratory. The objective of this study was to use SPME with GC-MS analysis as a method to detect insect biomarkers (Figure 1) as a tool for the identification of early insect infestation in stored grains, such as rice.
Figure 1.Characteristic VOC compounds produced by insects at the larvae stage.
Experimental
The HS-SPME method optimization was achieved using spiked rice samples obtained from a local market with undetectable GC-MS levels of studied analytes. During method development, fiber selectivity, extraction time (2, 5, 10, 15, 20 min), and temperature (30, 40, 50 and 60 °C) parameters were studied. For this purpose, 1 g of rice was spiked at 10 ng/g with 1 µL of a 10 µg/mL solution of analytes prepared in methanol. The HS-SPME-GC-MS method is summarized in Tables 1 and 2.
Results and Discussion
HS-SPME Method Optimization Procedure
Coating selectivity: A fiber selectivity study was performed using PDMS, DVB/PDMS, CAR/PDMS, and DVB/CAR/PDMS SPME fibers to evaluate the performance and effectiveness of each fiber coating chemistry on the headspace extraction of insect volatile biomarkers in a 10 ng/g spiked rice sample. The extraction conditions were as follows: equilibrium time of 2 min, extraction time of 10 min, and temperature of extraction of 40 °C, further sample preparation conditions are mentioned in the experimental section. Chromatographic biomarker profiles using different SPME coating chemistries are shown in Figure 2. It can be observed that CAR-PDMS on nitinol and DVB-CAR-PDMS exhibit better analyte response for the sample tested.

Figure 2.Chromatographic profile for selected biomarkers using four SPME coating chemistries. Sample: 1 g rice spiked at 10 ng/g of selected insect biomarkers. (* Fiber background)
The results of comparing different fibers are shown in Figure 3, which depicts the average response (area counts) for the different tested fibers
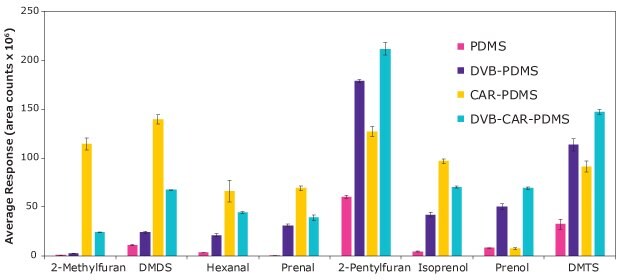
Figure 3.Evaluation of four SPME coating chemistries on the extraction of selected insect biomarkers.
Overall, CAR/PDMS fiber renders a good extraction performance for most of the analytes. Especially for small analytes, where the micropores present in the fiber retain and release these analytes efficiently. However, DVB-CAR-PDMS coating extracts more efficiently prenol in comparison to the rest of the analytes. This is due to the better interaction of this analyte with the DVB layer. Thus, CAR/PDMS on nitinol and DVB-CAR-PDMS on StableFlex (SF) were used for further HS-SPME method optimization.
The parameters for HS-SPME and GC/MS optimized methods are listed in Tables 1 and 2. Peak identifications were assigned using MS spectral matching against reference spectra in the Wiley and NIST libraries. Additionally, confirmatory identification was done by comparing the MS spectra of the sample with analytical standards.
Effect of extraction time: The influence of the extraction time was investigated in the range from 2 to 20 min. Figure 4 shows that the extraction efficiency for CAR-PDMS and DVB-CAR-PDMS increased as the extraction time increased up to 10 min, reaching the equilibrium for all the analytes. Thus, an extraction time of 10 min was selected for both coating chemistries.


Figure 4.Extraction time for selected insect biomarkers via HS-SPME-GC- MS using CAR-PDMS and CAR-DVB-PDMS SPME fibers. Mean values and standard deviation of analyte peak area (n=3). Sample: 1 g rice spiked at 10 ng/g of selected insect biomarkers.
Effect of extraction temperature: For the present study, the effect of the extraction temperature was examined in the range between 30 and 60 °C. The results shown in Figure 5 indicate that there was no significant effect on the analyte response for most of the analytes between 30 and 40 °C for both fiber chemistries. However, the peak area exhibited a slight decrease when the temperature increased up to 60 °C, possibly due to the desorption of the volatile analytes from the coating. Therefore, the extractions were carried out at 30 °C.


Figure 5.Extraction temperature for selected insect biomarkers via HS-SPME-GC-MS with CAR-PDMS and CAR-DVB-PDMS. Mean values and standard deviation of analyte peak area (n=3). Sample: 1 g rice spiked at 10 ng/g of selected insect biomarkers.
Recovery and Reproducibility
Table 3 depicts linearity, recovery, and reproducibility values using CAR-PDMS and DVB-CAR-PDMS fibers. Linearity was obtained through the construction of a multipoint calibration curve, at seven different concentration levels from 2.5 ng/g - 200 ng/g and using benzene-13C6 (10 ng/g) as an internal standard. The calibration curve for each analyte was prepared by adding proper volumes of standard solution and IS into SPME vials containing 1.0 g of rice. Excellent linearity and accuracy for all the analytes were observed for both SPME fibers in the studied calibration range. CAR-PDMS and DVB-CAR-PDMS fibers exhibit accuracy values of 61-103% and 77- 90%, respectively. Repeatability ≤ 10% RSD was observed for all the analytes for both fiber chemistries, and this was determined by analyzing 3 replicates of SPME extractions of rice samples spiked at 10 ng/g. As can be observed from Table 3, CAR-PDMS on nitinol exhibits higher extraction performance for all the analytes except prenol (recoveries: prenol 61%, other analytes 91-103%), which is likely due to stronger retention of the analyte in the micropores present in the structure of the fiber. Thus, CAR-PDMS is an excellent fiber choice for the detection of early volatile biomarkers in rice. However, DVB-CAR-PDMS can be used as a complementary fiber chemistry for the extraction of prenol.
Conclusion
An HS-SPME-GC-MS method has been developed for high-throughput analysis and detection of early volatile insect biomarkers in rice samples. Carboxen®-PDMS on nitinol fiber core was found to be most effective in the extraction and desorption of 2-methylfuran, DMDS, hexanal, prenal, 2-pentylfuran, isoprenol, and dimethyl trisulfide compared to DVB-CAR-PDMS on Stableflex™ fiber core. However, it presented low extraction performance only for prenol. Thus, CAR-PDMS is an excellent fiber choice for the detection of early volatiles indicating insect infestation in rice. However, DVB-CAR- PDMS can be used as a complementary fiber chemistry for the detection of prenol.
The HS-SPME-GC-MS method can be used in integrated pest management (IPM) programs as a fast and versatile monitoring approach/tool for the identification of early insect infestation in store grains such as rice.
Learn more about the SPME and applications.
To download the "SPME for GC" brochure, providing an overview on the technique, hints for method optimization and trouble shooting visit SigmaAldrich.com/SPME.
References
Zaloguj się lub utwórz konto, aby kontynuować.
Nie masz konta użytkownika?