Lithium Aminoborohydride (LAB) Reagents
Lithium aminoborohydride (LAB) reagents are a new class of powerful and selective reagents developed in the laboratory of Professor Bakthan Singaram at the University of California, Santa Cruz.
These reagents have reactivity comparable to lithium aluminum hydride (LAH). However, LABs have several advantages over LAH in that they are air-stable, nonpyrophoric, thermally stable, and hydrolyze only slowly in protic solvents above pH 4. Thus, LABs can perform in air virtually all of the transformations for which LAH is commonly used, and they offer significant advantages in safety, selectivity, ease of handling, and simple work-up procedures. LABs are capable of reducing a variety of functional groups, as summarized in Scheme 1.1
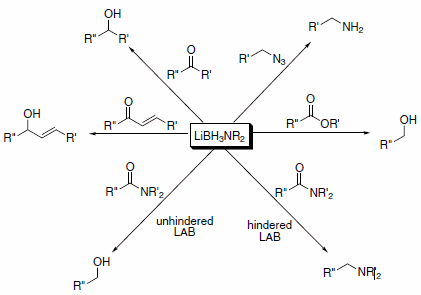
Scheme 1.Lithium aminoborohydride (LAB) reagents
In the case of N-alkyl lactams with an ester group, LAB can be used at reduced temperatures to selectively reduce the ester, leaving the lactam intact. At elevated temperatures, the lactam is also reduced to form the cyclic amine (Scheme 2).2
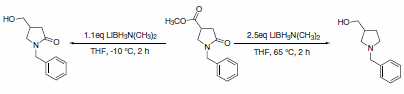
Scheme 2.Cyclic amine
In addition to hydride transfer, LABs can transfer the amine moiety, as in the case of reaction with halopyridines (Scheme 3)3 and primary alkyl methanesulfonates (Scheme 4)4. Alkyl methanesulfonates can be reduced to the corresponding alkanes with LAB reagents if Et3B is added in substoichiometric amounts (Scheme 5).4
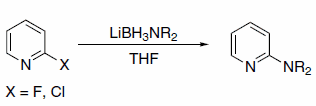
Scheme 3.Halopyridines
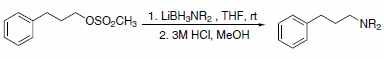
Scheme 4.Primary alkyl methanesulfonates
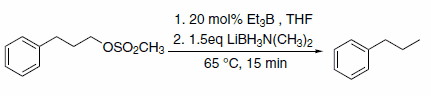
Scheme 5.Substoichiometric amounts
The LAB reagents’ ability to function as both a reducing agent and amination reagent allows the researcher to perform tandem amination-reduction reactions, as in the case of reaction with 2-halobenzonitriles.5 The LAB reagent activates the halo-benzonitrile toward nucleophilic attack by the amine moiety of the LAB reagent. This protocol is particularly effective with 2-fluorobenzonitrile (Scheme 6).
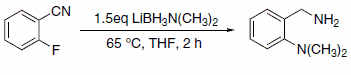
Scheme 6.2-fluorobenzonitrile
References
Zaloguj się lub utwórz konto, aby kontynuować.
Nie masz konta użytkownika?