Asymmetric Transfer Hydrogenation
The use of transfer hydrogenation to reduce alkenes, carboxyl groups, ketones, or imines has become very popular. Hashiguchi et al. reported the asymmetric transfer hydrogenation of ketones using a catalyst system comprised of ruthenium complexed with a chiral diamine ligand (RuCl(p-cymene)[(S,S)-Ts-DPEN], Scheme 5).1 Subsequently, this methodology was extended to include transfer hydrogenation of imines with low catalyst loadings, good yields, and excellent enantioselectivities of the desired products observed (Schemes 6 and 7). A variety of imines were subjected to these reaction conditions, with slight variations in the catalyst-ligand composition and/or solvent, leading to excellent yields and enantioselectivities of the functionalized amine heterocycles.2
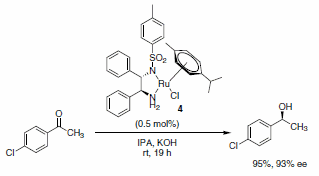
Scheme 5.
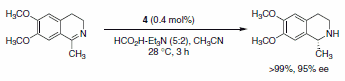
Scheme 6.

Scheme 7.
Matsumura and co-workers also accomplished the asymmetric transfer hydrogenation of α,β-acetylenic ketones using the RuCl(p-cymene) [(R,R)-Ts-DPEN] catalyst system. As shown in Scheme 8, the reduction to the propargylic alcohols occurs selectively without any competitive reaction with the alkynes.3
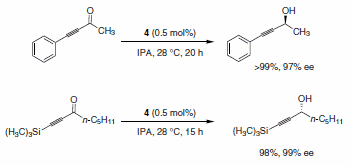
Scheme 8.
References
Zaloguj się lub utwórz konto, aby kontynuować.
Nie masz konta użytkownika?