Bispecific Monoclonal Antibody Analysis
Stacy Shollenberger1, Cory E. Muraco2
1HPLC Columns Tosoh Bioscience LLC, 2Technology and Workflow R&D
Introduction
More potent formats of monoclonal antibodies (mAbs), such as bispecific antibodies (bsAbs), are on the rise in the area of biotherapeutics. bsAbs recognize two different epitopes. This dual specificity increases the potency of these molecules compared to mAbs and expands the range of possible applications. bsAbs can be used to redirect T cells to tumor cells, block two different signaling pathways simultaneously, dually target different disease mediators, and deliver payloads to targeted sites. At this time, more than 50 bsAb products are currently undergoing clinical evaluation.1
Characterization of bsAbs is essential to ensuring product safety and efficacy. Size Exclusion Chromatography (SEC) coupled with Mass Spectrometry (MS) is increasingly being used to identify the accurate molecular mass of biomolecules, including bsAbs. SEC-MS, however, requires the use of mobile phases that do not contain high concentrations of non-volatile salts and the use of columns that do not exhibit column bleed, both of which will interfere with the MS signal response.
In this application, a Bispecific T cell Engager (BiTE®) consisting of two single-chain variable fragments (scFvs) recombinantly linked by a nonimmunogenic five-amino-acid chain (Figure 1) was analyzed by SEC-MS using a TSKgel® UP-SW3000, 2 μm column.
The ~55 kDa BiTE® and ~150 kDa parent mAbs were injected separately onto a TSKgel UP-SW3000 column coupled to a Q Exactive Plus mass spectrometer for molar mass determination. Figure 2 shows the (a) total ion chromatogram, (b) mass spectrum, and (c) deconvoluted mass spectrum of the BiTE®. A main peak can be seen at m/z 54,143; adjacent peaks at m/z 54,181, 54,219 and 54,086 correspond to different salt adducts.
Figure 3 shows the (a) total ion chromatogram, (b) mass spectrum, and (c) deconvoluted mass spectrum of one of the parent mAbs. A main peak can be seen at m/z 149,264; adjacent peaks at m/z 149,426, and 149,592 correspond to different glycoforms. Similar results (not shown) were obtained for the other parent mAb.
These results demonstrate accurate molar mass determination for the BiTE® and both parent mAbs utilizing a 20 mmol/L ammonium acetate, 10 mmol/L ammonium bicarbonate (pH 7.2) mobile phase with SEC-MS compatibility.

Figure 1.Formation of Bispecific T Cell Engager (BiTE®)
*SEC-MS analysis was performed by the Wistar Proteomics and Metabolomics Facility (Philadelphia, PA)
Prior to analysis, a blank injection was run in order to assess column bleed. Figure 4a shows the total ion chromatogram of a blank injection that was run on a new TSKgel® UP-SW3000 column. MS data indicates that there is no column bleed from the TSKgel® UP- SW3000 column prior to sample injection. Additionally, a blank injection was run between each of the sample injections in order to monitor sample carryover. Figure 4b shows the total ion chromatogram of a blank injection run between the BiTE® and parent mAb. No evidence of carryover can be seen in the run after sample injection. The lack of column bleed and carryover indicate that the TSKgel® UP-SW3000 column is suitable for use with MS.
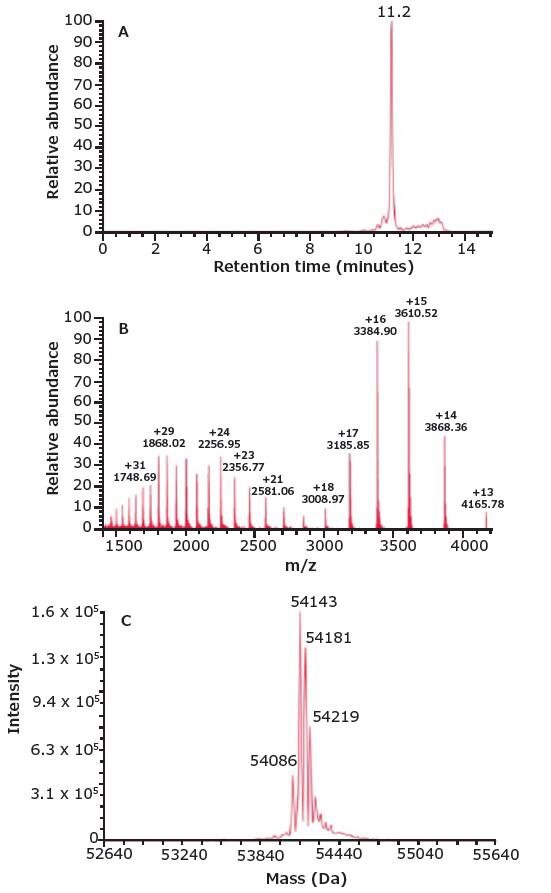
Figure 2.SEC-MS analysis of the BiTE®. Accurate molar mass of the BiTE® was calculated to be 54.1 kDa via SEC-MS .

Figure 3.SEC-MS Analysis of Parent mAb. Accurate molar mass of the parent mAb was calculated to be 149.3 kDa via SEC-MS

Figure 4.Column Bleed and Carryover Analysis. No column bleed or carryover was observed via MS total ion chromatogram.
Conclusion
The TSKgel® UP-SW3000, 2 μm SEC column can be used as a platform method for bispecific antibody accurate mass determination using SEC-MS. A MS compatible mobile phase under non-denaturing conditions was successfully used with the TSKgel® UP-SW3000 column. No signs of column bleed or sample carryover, which may interfere with MS signal response, were noted with the TSKgel® UP-SW3000 column.
References
Zaloguj się lub utwórz konto, aby kontynuować.
Nie masz konta użytkownika?