Y0000429
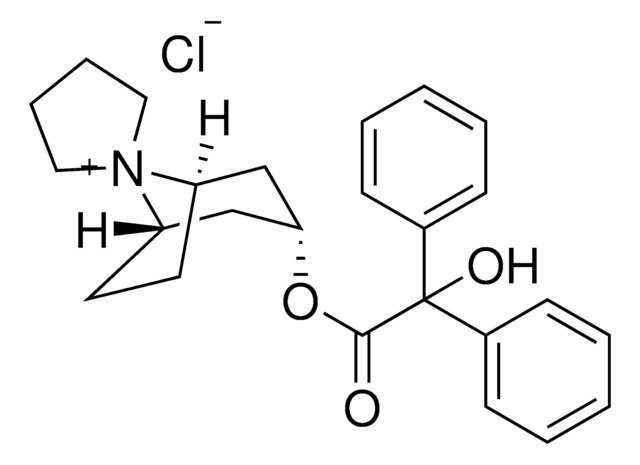
Trospium chloride
European Pharmacopoeia (EP) Reference Standard
About This Item
Polecane produkty
klasa czystości
pharmaceutical primary standard
rodzina API
trospium
producent / nazwa handlowa
EDQM
Zastosowanie
pharmaceutical (small molecule)
format
neat
temp. przechowywania
2-8°C
InChI
1S/C25H30NO3.ClH/c27-24(25(28,19-9-3-1-4-10-19)20-11-5-2-6-12-20)29-23-17-21-13-14-22(18-23)26(21)15-7-8-16-26;/h1-6,9-12,21-23,28H,7-8,13-18H2;1H/q+1;/p-1
Klucz InChI
RVCSYOQWLPPAOA-UHFFFAOYSA-M
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
Zastosowanie
Opakowanie
Inne uwagi
Choose from one of the most recent versions:
Certyfikaty analizy (CoA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej