K0133
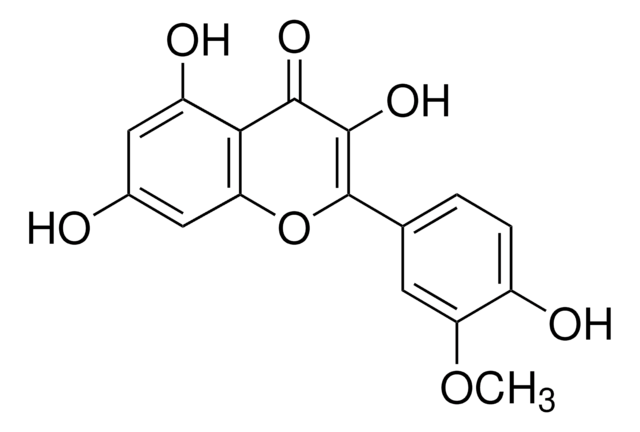
Kaempferol
≥90% (HPLC), powder
Sinónimos:
3,4′,5,7-Tetrahydroxyflavone, 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, Robigenin
About This Item
Productos recomendados
origen biológico
synthetic
Ensayo
≥90% (HPLC)
Formulario
powder
condiciones de almacenamiento
protect from light
color
yellow
mp
277 °C
solubilidad
ethanol: 20 mg/mL
DMSO: 50 mg/mL
temp. de almacenamiento
room temp
cadena SMILES
Oc1ccc(cc1)C2=C(O)C(=O)c3c(O)cc(O)cc3O2
InChI
1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H
Clave InChI
IYRMWMYZSQPJKC-UHFFFAOYSA-N
Información sobre el gen
human ... CDC2(983) , CDK5(1020) , CDK6(1021) , CYP1A2(1544) , CYP2C9(1559) , GSK3A(2931)
mouse ... Hexa(15211)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
Aplicación
- to check its potential effect as an antioxidant and neuroprotective agent against rotenone-induced Parkinson′s disease (PD) model in SH-S5Y5 cells
- to test its anti-inflammatory effect on lipopolysaccharide (LPS)-induced inflammatory injury in human aortic endothelial cells (HAECs)
- to study its apoptosis sensitizing effect on non-small cell lung cancer (NSCLC) cells by inhibiting nuclear factor erythroid 2-related factor 2 (Nrf2)
Acciones bioquímicas o fisiológicas
Envase
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico