15256
BSA+TMCS
for GC derivatization, LiChropur™, 93.0-97.0% (GC)
Sinónimos:
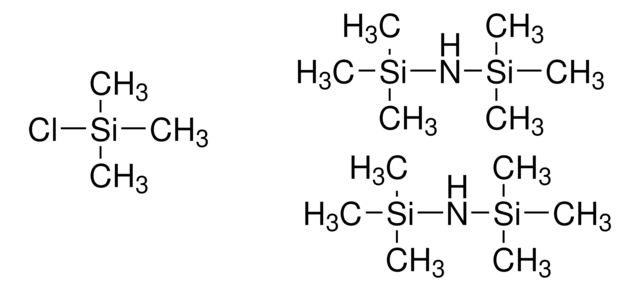
Bis(trimethylsilyl)acetamide + Trimethylchlorosilane
About This Item
Productos recomendados
grado
for GC derivatization
Nivel de calidad
Ensayo
93.0-97.0% (GC)
calidad
LiChropur™
composición
trimethylchlorosilane (minor component), 3.0-5.0% GC
idoneidad de la reacción
reagent type: derivatization reagent
reaction type: Silylations
técnicas
gas chromatography (GC): suitable
Descripción general
Aplicación
Características y beneficios
- BSA+TMCS has good solvent properties and can function as a silylation reagent without additional solvents.
- Alternatively, the mixture is very soluble in most commonly used silylation solvents.
- This combination is extremely sensitive to moisture and should be handled under dry conditions.
Otras notas
Información legal
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1A
Riesgos supl.
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
53.6 °F - closed cup
Punto de inflamabilidad (°C)
12 °C - closed cup
Equipo de protección personal
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico