907278
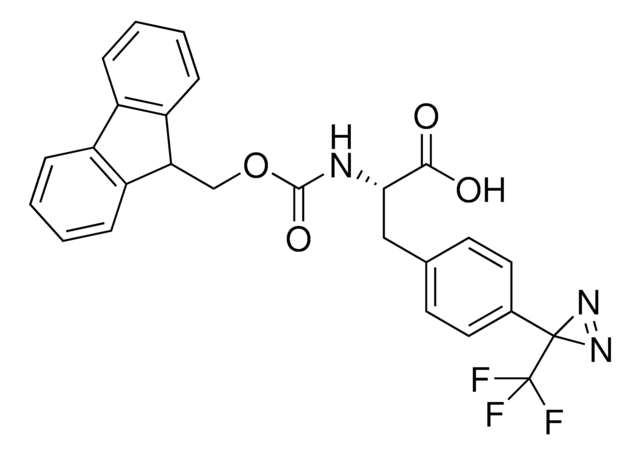
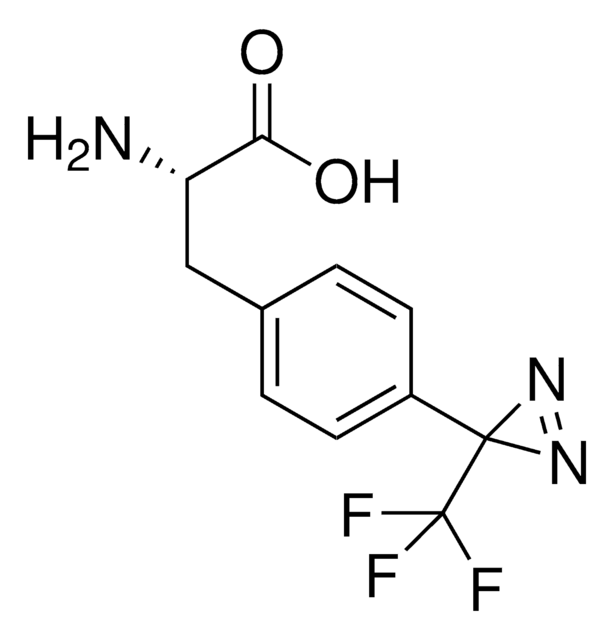
H-L-Photo-leucine HCl
≥98%
Sinónimos:
(S)-2-Amino-3-(3-methyl-3H-diazirin-3-yl)propanoic acid hydrochloride, (S)-2-Amino-3-(3H-diazirin-3-yl)butanoic acid hydrochloride, Diazirine amino acid, Photo-Leu, Photo-crosslinking amino acid, Photoprobe building block
About This Item
Productos recomendados
Ensayo
≥98%
Formulario
solid
idoneidad de la reacción
reaction type: solution phase peptide synthesis
disponibilidad
available only in USA
aplicaciones
peptide synthesis
temp. de almacenamiento
−20°C
Aplicación
Otras notas
for generation of homogeneous conjugates from wild-type antibodies
Mechanistic studies of a small-molecule modulator of SMN2 splicing
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Direct Interaction between an Allosteric Agonist Pepducin and the Chemokine Receptor CXCR4
Photo-leucine and photo-methionine allow identification of protein-?protein interactions in living cells
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Self-react. C
Código de clase de almacenamiento
5.2 - Organic peroxides and self-reacting hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico