The stereochemistry for Product 261696 is not defined. This product is not sold as a R or S isomer. The HPLC analysis note on the Product Detail Page for this product implies there may R or S isomers present in this product:
https://www.sigmaaldrich.com/technical-documents/chromatograms/hplc/hplc-astec-chirobiotic-tag/supelco/g004407
Therefore, it is possible stereoisomers may be present in this product.
261696
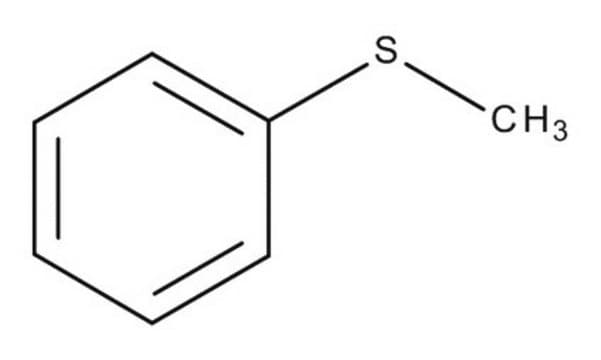
Methyl phenyl sulfoxide
≥97%
About This Item
Productos recomendados
Quality Level
assay
≥97%
form
crystals
refractive index
n20/D 1.5775 (lit.)
bp
139-140 °C/14 mmHg (lit.)
mp
26-29 °C (lit.)
functional group
sulfoxide
storage temp.
2-8°C
SMILES string
CS(=O)c1ccccc1
InChI
1S/C7H8OS/c1-9(8)7-5-3-2-4-6-7/h2-6H,1H3
InChI key
JXTGICXCHWMCPM-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
186.8 °F - closed cup
flash_point_c
86 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Chromatograms
application for HPLC-
MethylPhenylsulfoxide is racemic or enantiomer
1 answer-
Helpful?
-
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico