156671
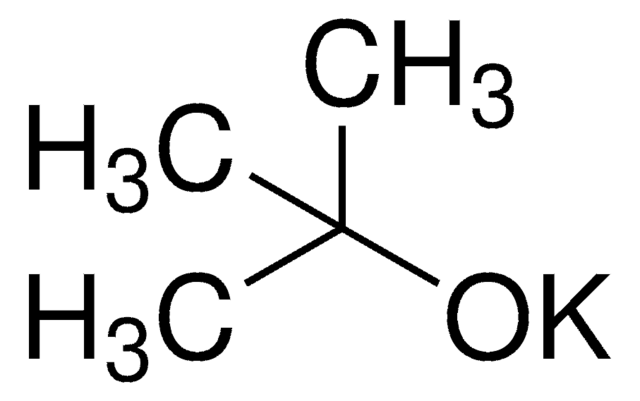
Potassium tert-butoxide
reagent grade, ≥98%
Sinónimos:
Potassium T-butoxide
About This Item
Productos recomendados
grade
reagent grade
Quality Level
vapor pressure
1 mmHg ( 220 °C)
assay
≥98%
form
solid
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
256-258 °C (dec.) (lit.)
greener alternative category
SMILES string
[K+].CC(C)(C)[O-]
InChI
1S/C4H9O.K/c1-4(2,3)5;/h1-3H3;/q-1;+1
InChI key
LPNYRYFBWFDTMA-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
It can also be used:
- To synthesize aliphatic and aromatic amides from corresponding esters and amines.
- As a base in the intramolecular cyclization of aryl ethers, amines, and amides.
- As a catalyst to prepare styrene derivatives from aryl halides and alkenes by Mizoroki-Heck reaction.
tert-Butoxide-Assisted Amidation of Esters under Green Conditions
Potassium tert-butoxide may be used as a base in the intramolecular cyclization of iodo arene to afford benzopyran via microwave method of synthesis.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Sol. 1 - Self-heat. 2 - Skin Corr. 1A
supp_hazards
Storage Class
4.2 - Pyrophoric and self-heating hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico