H9631
DL-α-Hydroxystearic acid
≥99%
Sinónimos:
D,L-2-hydroxystearic acid, 2-Hydroxyoctadecanoic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C18H36O3
Número de CAS:
Peso molecular:
300.48
Número MDL:
Código UNSPSC:
12352211
ID de la sustancia en PubChem:
NACRES:
NA.25
Productos recomendados
origen biológico
synthetic (organic)
Nivel de calidad
Ensayo
≥99%
Formulario
powder
grupo funcional
carboxylic acid
tipo de lípido
saturated FAs
Condiciones de envío
ambient
temp. de almacenamiento
2-8°C
cadena SMILES
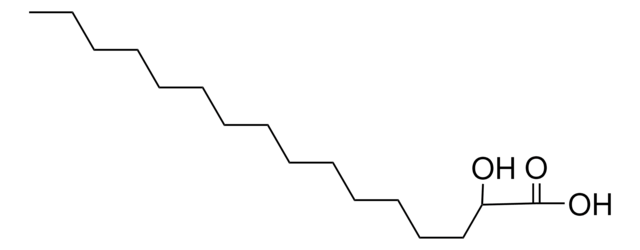
CCCCCCCCCCCCCCCCC(O)C(O)=O
InChI
1S/C18H36O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17(19)18(20)21/h17,19H,2-16H2,1H3,(H,20,21)
Clave InChI
KIHBGTRZFAVZRV-UHFFFAOYSA-N
Categorías relacionadas
Acciones bioquímicas o fisiológicas
DL-α-Hydroxystearic acid is a mixture of D and L-α-hydroxystearic acid (2-Hydroxyoctadecanoic acid) enantiomers. α-Hydroxystearic may be used in studies on the properties and metabolism of α-hydroxylated (2-hydroxylated) medium chain fatty acids.
Envase
Sealed ampule.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
M Salim-Hanna et al.
Lipids, 24(8), 750-752 (1989-08-01)
Combined--but not individual--microsomal and supernatant fractions obtained from rat brains not only consume oxygen but also provoke emission from added chlorophyll. These results are consistent with literature data (Levis and Mead, J. Biol. Chem. 239, 77 [1964]) for trapping of
Samanta R Zanetti et al.
Biochimie, 92(12), 1778-1786 (2010-09-14)
Sphingolipids from rodent testis and spermatozoa are known to contain non-hydroxylated (N-) and 2-hydroxylated (2-OH) very-long-chain polyunsaturated fatty acids (VLCPUFA). In this study, the contribution of species with each type of fatty acids to the total ceramides (Cer) and sphingomyelins
S Sonnino et al.
Chemistry and physics of lipids, 69(2), 95-104 (1994-02-01)
GM1 ganglioside containing a hydroxylated fatty acid moiety, GM1(OH), was synthesized starting from lyso-GM1 and D-(+)-2-hydroxystearic acid. The aggregative, geometrical and distribution properties of GM1(OH) were compared with those of stearic acid containing GM1 ganglioside; laser light scattering measurements, differential
Voradanu Visetvichaporn et al.
International journal of pharmaceutics, 573, 118772-118772 (2019-11-26)
HL235 is a new cathepsin K inhibitor designed and synthesized to treat osteoporosis. Since HL235 has poor aqueous solubility, a self-microemulsifying drug delivery system (SMEDDS) was formulated to enhance its oral bioavailability. A solubility study of HL235 was performed to
Conrad D Lendrum et al.
Langmuir : the ACS journal of surfaces and colloids, 27(8), 4430-4438 (2011-03-23)
2-Hydroxyacids display complex monolayer phase behavior due to the additional hydrogen bonding afforded by the presence of the second hydroxy group. The placement of this group at the position α to the carboxylic acid functionality also introduces the possibility of
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico