Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
PHR1422
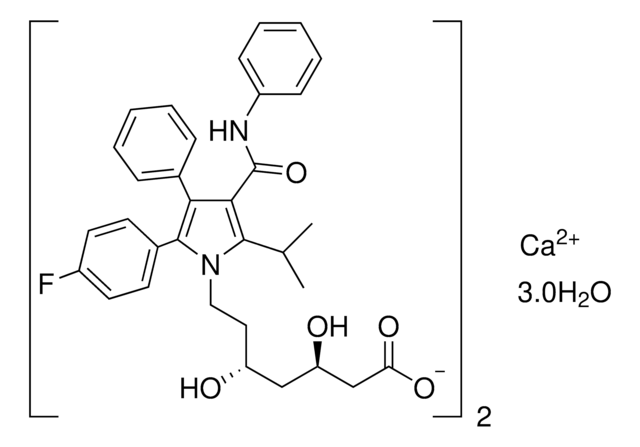
Atorvastatin Calcium
Pharmaceutical Secondary Standard; Certified Reference Material
Sinónimos:
Atorvastatin hemicalcium salt sesquihydrate, 1H-Pyrrole-1-heptanoic acid, [R-(R*,R*)]-2-(4-flurophenyl)-β,δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl], calcium salt (2:1)
About This Item
Productos recomendados
grado
certified reference material
pharmaceutical secondary standard
Nivel de calidad
Agency
traceable to Ph. Eur. Y0001327
traceable to USP 1044516
familia API
atorvastatin
CofA
current certificate can be downloaded
técnicas
HPLC: suitable
gas chromatography (GC): suitable
aplicaciones
pharmaceutical (small molecule)
Formato
neat
temp. de almacenamiento
2-30°C
cadena SMILES
[Ca+2].Fc5ccc(cc5)c6[n](c(c(c6c8ccccc8)C(=O)Nc7ccccc7)C(C)C)CC[C@@H](O)C[C@@H](O)CC(=O)O.Fc1ccc(cc1)c2[n](c(c(c2c4ccccc4)\C(=N\c3ccccc3)\[O-])C(C)C)CC[C@@H](O)C[C@@H](O)CC(=O)[O-].O.O.O
InChI
1S/2C33H35FN2O5.Ca.3H2O/c2*1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40;;;;/h2*3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40);;3*1H2/q;;+2;;;/p-2/t2*26-,27-;;;;/m11..../s1
Clave InChI
SHZPNDRIDUBNMH-NIJVSVLQSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Aplicación
Nota de análisis
Otras notas
Nota al pie de página
Analito
Producto relacionado
columna analítica
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Carc. 2 - Repr. 1B
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Chromatograms
application for HPLC-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Helpful?
-
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdfHelpful?
-
-
I have made a stock solitution in DMSO. At what temperature should I store it and what is the shelf life?
1 answer-
The stability of this product in DMSO has not been determined. However, various sources recommend storing stock solutions in DMSO at -20°C for 1 month and at -80°C for 1 year. As a general rule, avoid repeated freeze/thaw cycles. This information has not been validated.
Helpful?
-
-
Buenos días, como debe reconstituirse el producto: Atorvastatin Calcium, PHR1422? Muchas gracias, Aina
1 answer-
The following solubility characteristics are given in the monograph of the European Pharmacopoeia: practically insoluble in water, very slightly soluble to very soluble in ethanol (96 percent), freely soluble to very soluble in methanol, practically insoluble to freely soluble in methylene chloride, and freely soluble in dimethyl sulfoxide.
Helpful?
-
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico