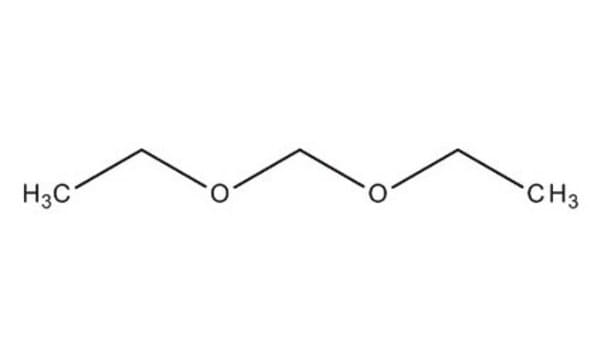
D134651
Dimethoxymethane
ReagentPlus®, 99%
Sinónimos:
Formaldehyde dimethyl acetal, Methylal
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH2(OCH3)2
Número de CAS:
Peso molecular:
76.09
Beilstein/REAXYS Number:
1697025
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Productos recomendados
vapor density
2.6 (vs air)
Quality Level
vapor pressure
6.38 psi ( 20 °C)
product line
ReagentPlus®
assay
99%
form
liquid
autoignition temp.
459 °F
expl. lim.
17.6 %
refractive index
n20/D 1.354 (lit.)
bp
41-42 °C (lit.)
mp
−105 °C (lit.)
density
0.86 g/mL at 25 °C (lit.)
SMILES string
COCOC
InChI
1S/C3H8O2/c1-4-3-5-2/h3H2,1-2H3
InChI key
NKDDWNXOKDWJAK-UHFFFAOYSA-N
Categorías relacionadas
General description
Dimethoxymethane (DMM, methylal) is a biodegradable dimethyl acetal. It can be synthesized by acid catalyzed condensation of formaldehyde with methanol. It is amphiphilic in nature with low viscosity, surface tension and boiling point. It is a flammable, highly volatile solvent with good dissolving power. DMM is considered as a potential alternative fuel and fuel additive due to its high oxygen content and its ability to enhance the combustion characteristics of diesel and petrol. Its thermal diffusivity has been determined by photoacoustic method. Analysis of the molecular structure of DMM by electron diffraction technique shows that it has C2 symmetry with a gauche-gauche conformation.
Application
Dimethoxymethane (Formaldehyde dimethyl acetal) may be used in the synthesis of methoxymethyl (MOM) ethers. It may also be used as an external cross-linker to form microporous polymers.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 2
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
-0.4 °F - closed cup
flash_point_c
-18 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
The molecular structure of dimethoxymethane.
Astrup EE.
Acta Chemica Scandinavica, 27(9), 3271-3276 (1973)
A photoacoustic study on Dimethoxymethane.
Bama GK and Ramachandran K.
AIP Conference Proceedings, 1004, 191-191 (2008)
A new strategy to microporous polymers: knitting rigid aromatic building blocks by external cross-linker.
Li B, et al.
Macromolecules, 44(8), 2410-2414 (2011)
An efficient protocol for the preparation of MOM ethers and their deprotection using zirconium (IV) chloride.
Sharma GVM, et al.
Tetrahedron Letters, 45(50), 9229-9232 (2004)
Jenn-Huei Lii et al.
Carbohydrate research, 340(5), 853-862 (2005-03-23)
The rotational barrier for a methyl group at the end of an anomeric system is sometimes lower than we might have anticipated. Thus, in the trans-trans conformation of dimethoxymethane, the barrier to methyl rotation is calculated (B3LYP/6-311++G(2d,2p)) to be 2.22
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico