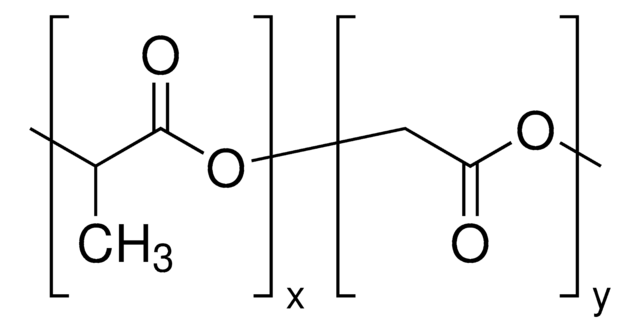
Product 430471 has a dodecyl (12 carbon) ester on one end of the polymer chain and a hydroxyl end group on the other.
430471
Poly(D,L-lactide-co-glycolide)
ester terminated, Mw 50,000-75,000
Sinónimos:
Lactel® B6006-1, PLGA
About This Item
Productos recomendados
Quality Level
form
amorphous
feed ratio
lactide:glycolide 85:15
mol wt
Mw 50,000-75,000
degradation timeframe
<6 months
viscosity
0.55-0.75 dL/g, 0.1 % (w/v) in chloroform(25 °C)
transition temp
Tg 45-50 °C
solubility
ethyl acetate, chloroform, acetone and THF: soluble
storage temp.
2-8°C
SMILES string
OCC(O)=O.CC(O)C(O)=O
InChI
1S/C3H6O3.C2H4O3/c1-2(4)3(5)6;3-1-2(4)5/h2,4H,1H3,(H,5,6);3H,1H2,(H,4,5)
InChI key
XBBVURRQGJPTHH-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
Features and Benefits
Physical form
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Interest in utilizing biodegradable polymers for biomedical applications has grown since the 1960s.
Synthetic aliphatic polyesters dominate resorbable biomaterials in clinical use.
Synthetic aliphatic polyesters dominate resorbable biomaterials in clinical use.
Synthetic aliphatic polyesters dominate resorbable biomaterials in clinical use.
-
What are the end groups of Product No. 430471 (Poly(DL-lactide-co-glycolide)?
1 answer-
Helpful?
-
-
What is the tensile strength of Product No. 403471 Poly(DL-lactide-co-glycolide)?
1 answer-
Product 430471 has a tensile strength of 6000 - 8000 psi, with an elongation of 3-10%.
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
What is the density of Product No. 430471 Poly(DL-lactide-co-glycolide)?
1 answer-
According to the manufacturer, product 430471 has a theoretical density of 1.27g/mL.
Helpful?
-
-
Is Product No. 403471 a resorbable polymer?
1 answer-
Yes, Product No. 430471 has a resorption time of 5-6 months.
Helpful?
-
-
How is this Product No. 430471 synthesized (Poly(DL-lactide-co-glycolide)?
1 answer-
To synthesize product 430471, 1-dodecanol is used as an initiator and stannous octoate is used as a catalyst to start the ring opening polymerization of D,L-lactide and glycolide monomers.
Helpful?
-
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico