All Photos(1)
About This Item
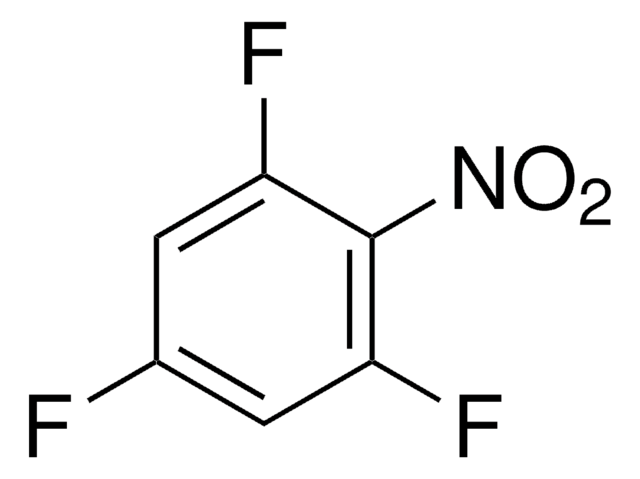
Linear Formula:
F2C6H3NO2
CAS Number:
Molecular Weight:
159.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
refractive index
n20/D 1.494 (lit.)
bp
91-92 °C/11 mmHg (lit.)
density
1.503 g/mL at 25 °C (lit.)
functional group
fluoro
nitro
SMILES string
[O-][N+](=O)c1c(F)cccc1F
InChI
1S/C6H3F2NO2/c7-4-2-1-3-5(8)6(4)9(10)11/h1-3H
InChI key
SSNCMIDZGFCTST-UHFFFAOYSA-N
General description
2,6-Difluoronitrobenzene is an organic building block. Molecular structure, conformation and potential to internal rotation of 2,6-difluoronitrobenzene been studied by gas-phase electron diffraction (GED), MP2 ab initio, and by B3LYP density functional calculations.
Application
2,6-Difluoronitrobenzene may be used in the preparation of secondary amine precursors, required for the synthesis of two families of nitric oxide donors.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
190.4 °F - closed cup
Flash Point(C)
88 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Brandon Curtis et al.
Bioorganic & medicinal chemistry, 21(5), 1123-1135 (2013-02-05)
Atherosclerosis, a leading cause of death worldwide, is associated with the excessive proliferation of vascular smooth muscle cells. Nitrogen monoxide, more commonly known as nitric oxide, inhibits this uncontrolled proliferation. Herein we report the preparation of two families of nitric
Olga V Dorofeeva et al.
The journal of physical chemistry. A, 112(22), 5002-5009 (2008-05-09)
3,5-Difluoronitrobenzene (3,5-DFNB) and 2,6-difluoronitrobenzene (2,6-DFNB) have been studied by gas-phase electron diffraction (GED), MP2 ab initio, and by B3LYP density functional calculations. Refinements of r h1 and r e static and r h1 dynamic GED models were carried out for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service