288365
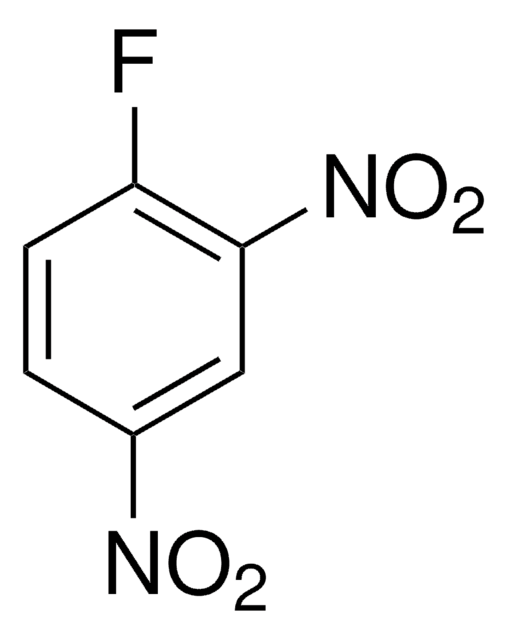
3,4-Difluoronitrobenzene
99%
Synonym(s):
1,2-Difluoro-4-nitrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
F2C6H3NO2
CAS Number:
Molecular Weight:
159.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.509 (lit.)
bp
76-80 °C/11 mmHg (lit.)
density
1.437 g/mL at 25 °C (lit.)
functional group
fluoro
nitro
SMILES string
[O-][N+](=O)c1ccc(F)c(F)c1
InChI
1S/C6H3F2NO2/c7-5-2-1-4(9(10)11)3-6(5)8/h1-3H
InChI key
RUBQQRMAWLSCCJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The experimental and computational thermochemical study of 3,4-difluoronitrobenzene was studied.
Application
3,4-Difluoronitrobenzene was used in the preparation of xanthones and acridones.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
177.8 °F - closed cup
Flash Point(C)
81 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Oludotun A Phillips et al.
Journal of enzyme inhibition and medicinal chemistry, 35(1), 1471-1482 (2020-07-09)
Oxazolidinone hydroxamic acid derivatives were synthesised and evaluated for inhibitory activity against leukotriene (LT) biosynthesis in three in vitro cell-based test systems and on direct inhibition of recombinant human 5-lipoxygenase (5-LO). Thirteen of the 19 compounds synthesised were considered active ((50%
Manuel A V Ribeiro da Silva et al.
The journal of physical chemistry. B, 114(40), 12914-12925 (2010-09-24)
This work reports the experimental and computational thermochemical study performed on three difluorinated nitrobenzene isomers: 2,4-difluoronitrobenzene (2,4-DFNB), 2,5-difluoronitrobenzene (2,5-DFNB), and 3,4-difluoronitrobenzene (3,4-DFNB). The standard (p° = 0.1 MPa) molar enthalpies of formation in the liquid phase of these compounds were
Synthesis of heterocyclic compounds via nucleophilic aroylation catalyzed by imidazolidenyl carbene.
Yumiko Suzuki et al.
Chemical & pharmaceutical bulletin, 54(12), 1653-1658 (2006-12-02)
Xanthones and acridones were synthesized from 3,4-difluoronitrobenzene and 2-fluorobenzaldehydes in two or three steps. The key step was nucleophilic aroylation catalyzed by imidazolidenyl carbene. The nucleophilic aroylation of 3,4-difluoronitrobenzene afforded 2,2'-difluoro-4-nitrobenzophenones. The cyclization of the difluorobenzophenones with O-nucleophile and N-nucleophile
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service