All Photos(2)
About This Item
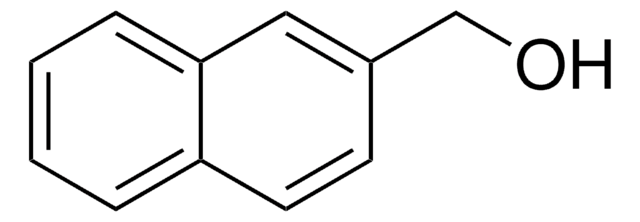
Linear Formula:
C10H7CH2OH
CAS Number:
Molecular Weight:
158.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
301 °C/715 mmHg (lit.)
mp
61-63 °C (lit.)
SMILES string
OCc1cccc2ccccc12
InChI
1S/C11H10O/c12-8-10-6-3-5-9-4-1-2-7-11(9)10/h1-7,12H,8H2
InChI key
PBLNHHSDYFYZNC-UHFFFAOYSA-N
Related Categories
General description
3′-Phosphoadenylyl sulfate dependent sulfation of 1-naphthalenemethanol catalyzed by isoenzyme of arylsulfotransferase has been studied. 1-Naphthalenemethanol is an intermediate photolysis product of 1-napthaleneacetic acid.
Application
1-Naphthalenemethanol was used to investigate UV photooxidation of 1-methylnaphthalene in the presence of dry or humid air.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
UV Light Induced Transformation of 1-Methylnaphthalene in the Presence of Air and Its Implications for Contaminants Research.
Feng Y-L, et al.
Journal of Environmental Protection, 3(11), 1519-1531 (2012)
Arylsulfotransferase IV catalyzed sulfation of 1-naphthalenemethanol.
M W Duffel et al.
Advances in experimental medicine and biology, 197, 415-422 (1986-01-01)
Photodecomposition of 1-naphthaleneacetic acid.
Crosby DG and Tang C-S.
Journal of Agricultural and Food Chemistry, 17(6), 1291-1293 (1969)
M W Duffel et al.
Analytical biochemistry, 183(2), 320-324 (1989-12-01)
An assay procedure for purified aryl sulfotransferase is described. The method utilizes isocratic paired-ion reverse-phase HPLC analysis of adenosine-3',5'-diphosphate formed in the reaction. Evaluation of the assay procedure was carried out with 1-naphthalene-methanol as a model substrate for purified rat
Danlei Wang et al.
Chemico-biological interactions, 315, 108905-108905 (2019-11-26)
Mineral oils are widely applied in food production and processing and may contain polycyclic aromatic hydrocarbons (PAHs). The PAHs that may be present in mineral oils are typically alkylated, and have been barely studied. Metabolic oxidation of the aromatic ring
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service