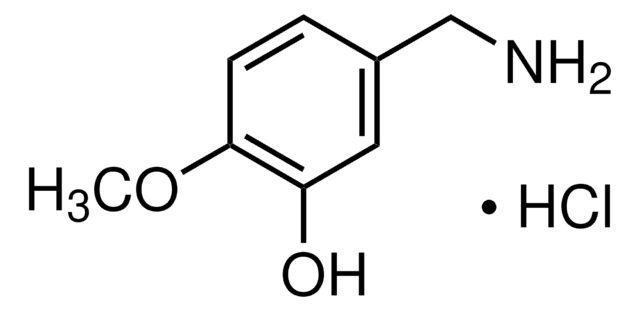
858781
3,4-Dihydroxybenzylamine hydrobromide
98%
Sinónimos:
4-(Aminomethyl)catechol hydrobromide, DHBA hydrobromide
About This Item
Productos recomendados
Quality Level
assay
98%
form
crystals
mp
184-186 °C (lit.)
SMILES string
Br.NCc1ccc(O)c(O)c1
InChI
1S/C7H9NO2.BrH/c8-4-5-1-2-6(9)7(10)3-5;/h1-3,9-10H,4,8H2;1H
InChI key
BVFZTXFCZAXSHN-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
<li><strong>Oxidative Polymerization of 3,4-Dihydroxybenzylamine:</strong> 3,4-Dihydroxybenzylamine is used in the synthesis of poly[3,4-dihydroxybenzylamine] (PDHBA) by oxidative polymerization, exploring its application as a lower homolog of dopamine for potential use in synthetic pathways and materials science (Petran et al., 2023).</li>
<li><strong>Detection of Urinary Free Metanephrines:</strong> Utilizing 3,4-Dihydroxybenzylamine hydrobromide as an internal standard, this research enhances the detection accuracy of urinary free metanephrines for diagnosing pheochromocytomas and paragangliomas, showcasing its importance in clinical diagnostic applications (Wang et al., 2020).</li>
<li><strong>Development of HPLC-ECD Method:</strong> A study developed an HPLC-ECD method using 3,4-Dihydroxybenzylamine as an internal standard for the analysis of vitamin C in plasma, demonstrating the chemical’s utility in enhancing analytical methodologies in biochemical research (Clark and Frank, 2016).</li>
<li><strong>Fluorescence Analysis of Catecholamines:</strong> 3,4-Dihydroxybenzylamine is used as an internal standard to determine catecholamines and related compounds in rat brain tissue, underlining its application in neurochemical analysis and research (Fonseca et al., 2017).</li>
</ul>
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico