All Photos(1)
About This Item
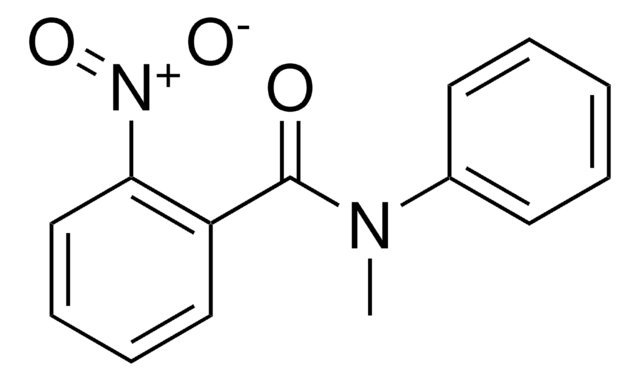
Linear Formula:
CH3NHC(=NH)NHNO2
CAS Number:
Molecular Weight:
118.09
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
form
solid
Quality Level
contains
~25% water
mp
153-155 °C (lit.)
SMILES string
CNC(=N)N[N+]([O-])=O
InChI
1S/C2H6N4O2/c1-4-2(3)5-6(7)8/h1H3,(H3,3,4,5)
InChI key
XCXKNNGWSDYMMS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Methyl-3-nitroguanidine (MNG) is formed as intermediate during the production of hydroxyl radical during 1-methyl-3-nitro-1-nitrosoguanidine (MNNG) - induced gastric cancer in the xanthine oxidase system. MNG has been referred by the U.S. Air Force Armament Laboratory for use in explosive formulations. MNG is formed during nucleophilic attack by H2O2 on the nitroso nitrogen of MNNG. MNG is non-carcinogenic analog of N-methyl-N′-nitro-N-nitrosoguanidine (direct-acting carcinogen).
Application
1-Methyl-3-nitroguanidine (N-methyl-N′-nitroguanidine) was used in the synthesis of clothianidin hapten.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Sol. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E R Kinkead et al.
Toxicology and industrial health, 9(3), 457-477 (1993-05-01)
Currently, N-methyl-N'-nitroguanidine (MNG) is being considered by the U.S. Air Force Armament Laboratory for use in explosive formulations. A mammalian toxicity profile has been performed which includes the analysis of chemical impurities and an assessment of the potential for the
Shuji Ohno et al.
Toxicology letters, 322, 32-38 (2020-01-11)
Neonicotinoids (NNs), a widely used class of systemic pesticides, are regarded as exhibiting selective toxicity in insects. However, NNs are suspected of exerting adverse effects on mammals as well, including humans. To date, only adult male animal models have been
A W Hsie et al.
Molecular toxicology, 1(2-3), 217-234 (1987-04-01)
Previously, we have shown that Chinese hamster ovary (CHO) cells are useful for quantifying chemical-induced gene mutations. We have defined the conditions of a Multiplex CHO System which permits determination of mutagen-induced chromosome aberration, and sister chromatid exchange (SCE) in
Tomiko Mikuni et al.
Free radical research, 36(6), 641-647 (2002-08-16)
We have examined the mechanism of 1-methyl-3-nitro-1-nitrosoguanidine (MNNG)-induced gastric cancer with respect to the production of hydroxyl free radical (OH). Nucleophilic attack by H2O2 on the nitroso group of MNNG produces 1-methyl-3-nitroguanidine (MNG) and the intermediate peroxynitric acid (ONOOH), which
Akihisa Abe et al.
Microbiological research, 162(2), 130-138 (2006-03-08)
The viable but nonculturable (VBNC) suppression mutant formed platable cells at low temperature stress after inoculation in artificial seawater (ASW). Suppression subtractive hybridization was used to identify differentially expressed genes among cDNAs of the VBNC suppression mutant and the wild-type
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service