M3205
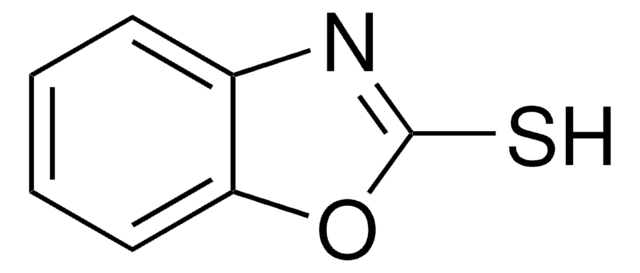
2-Mercaptobenzimidazole
98%
Synonym(s):
1,3-Dihydro-2H-benzimidazole-2-thione, 2-Benzimidazolethiol
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Empirical Formula (Hill Notation):
C7H6N2S
CAS Number:
Molecular Weight:
150.20
Beilstein:
119867
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
300-304 °C (lit.)
SMILES string
S=C1Nc2ccccc2N1
InChI
1S/C7H6N2S/c10-7-8-5-3-1-2-4-6(5)9-7/h1-4H,(H2,8,9,10)
InChI key
YHMYGUUIMTVXNW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Mercaptobenzimidazole is a good corrosion inhibitor as it reduces the corrosion rate of metals.
Application
2-Mercaptobenzimidazole can be used:
- To prepare 2-benzimidazolylthioacetophenones by reacting with aromatic ketones, which is a key intermediate for the synthesis of thiazolo[3,2-a]benzimidazoles.
- To synthesize S-arylated 2-mercapto-benzimidazoles via S-arylation with substituted aryl iodides using CuI and 1,10-phenanthroline.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 3 - Skin Sens. 1A - STOT RE 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S-arylation of mercaptobenzimidazoles using Cu (I) catalysts-experimental and theoretical observations
Sekar R, et al.
Tetrahedron Letters, 52(26), 3347-3352 (2011)
Yuqin Li et al.
Colloids and surfaces. B, Biointerfaces, 104, 311-317 (2013-01-22)
The interaction of 2-mercaptobenzimidazole (MBI) with human serum albumin (HSA) was studied in vitro by equilibrium dialysis under normal physiological conditions. This study used fluorescence, ultraviolet-visible spectroscopy (UV-vis), Fourier transform infrared (FT-IR), circular dichroism (CD) and Raman spectroscopy, atomic force
Edésio F C Alcântara et al.
Journal of colloid and interface science, 311(1), 1-7 (2007-04-10)
The compound 2-mercaptobenzimidazole (MBI) was attached onto a silica gel surface by homogeneous and heterogeneous routes. Both silica modification methodologies resulted in similar products, named SiM(hom) and SiM(het), respectively. These materials were characterized by surface area, infrared, thermogravimetry, and 13C
D M Manohar et al.
Water research, 36(6), 1609-1619 (2002-05-09)
The 2-mercaptobenzimidazole loaded natural clay was prepared for the removal of Hg(II) from aqueous media. Adsorption of the metal ions from aqueous solution as a function of solution concentration, agitation time, pH, temperature, ionic strength, particle size of the adsorbent
Kazue Sakemi et al.
Archives of toxicology, 76(12), 682-691 (2002-11-27)
2-Mercaptobenzimidazole (MBI), a rubber antioxidant, is known to exhibit potent thyroid toxicity in rats, whereas its methylated derivatives are much less toxic. To characterize this methyl-substituent effect on the thyroid toxicity of MBI, comparative toxicokinetic analyses have been conducted in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service