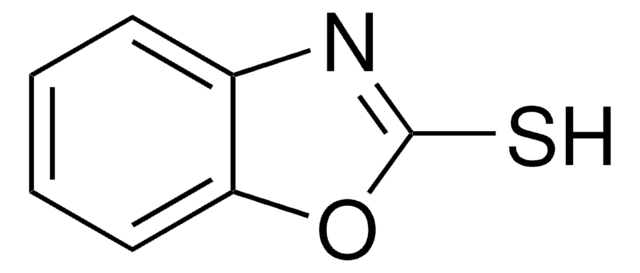
301507
2-Mercapto-1-methylimidazole
≥99%
Synonym(s):
Methimazole, 1-Methyl-2-imidazolethiol, 2-Mercapto-1-methylimidazole
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H6N2S
CAS Number:
Molecular Weight:
114.17
Beilstein:
108646
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
mp
144-147 °C (lit.)
SMILES string
CN1C=CNC1=S
InChI
1S/C4H6N2S/c1-6-3-2-5-4(6)7/h2-3H,1H3,(H,5,7)
InChI key
PMRYVIKBURPHAH-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544) , TPO(7173)
Looking for similar products? Visit Product Comparison Guide
General description
Sensitive detection of 2-mercapto-1-methylimidazole by Au-Ag-Au double shell nanoparticles-based localized surface plasmon resonance and surface-enhanced Raman scattering biosensor was reported.
Application
2-Mercapto-1-methylimidazole was employed as hydrophobic charge-induction chromatography ligand for antibody purification. It was also used in preparation of nitrile functionalized methimazole-based room temperature ionic liquids.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Repr. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hui-Li Lu et al.
Journal of chromatography. A, 1278, 61-68 (2013-01-23)
Hydrophobic charge-induction chromatography (HCIC) is a novel technology for antibody purification. The ligand densities and pore properties of HCIC resins have significant effects on the separation behavior of protein, however, the understandings are quite limited. In the present work, new
Xue Liao et al.
Talanta, 117, 203-208 (2013-11-12)
In this paper, Au-Ag-Au double shell nanoparticles were prepared based on the reduction of the metal salts HAuCl4 and AgNO3 at the surface of seed particles. Due to the synergistic effect between Au and Ag, the hybrid nanoparticles are particularly
Amal I Siriwardana et al.
The Journal of organic chemistry, 75(24), 8376-8382 (2010-11-18)
The alkylation reaction of 2-mercapto-1-methylimidazole 1b with 2-chloroacetonitrile and 2-chloropropionitrile produced S-alkyl methimazole chlorides 2a and 2b which were subjected to anion metathesis with lithium bis(trifluoromethanesulfonyl)amide, LiNTf(2), to afford nitrile functionalized methimazole-based room temperature ionic liquids 3a and 3b in
F S Boretti et al.
Journal of veterinary internal medicine, 27(2), 377-381 (2013-02-13)
Transdermal methimazole is an acceptable alternative to oral treatment for hyperthyroid cats. There are, however, no studies evaluating the duration of T4 suppression after transdermal methimazole application. Such information would be valuable for therapeutic monitoring. To assess variation in serum
Duygu Yeniceli et al.
Electrophoresis, 34(3), 463-470 (2012-11-20)
A selective and low-cost CD-MEKC method under acidic conditions was developed for investigating the N-oxygenation of tamoxifen (TAM) by flavin-containing monooxygenases (FMOs). The inhibitory effects of methimazole (MMI), nicotine and 5,6-dimethylxanthenone-4-acetic acid (DMXAA) on the given FMO reaction were also
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service