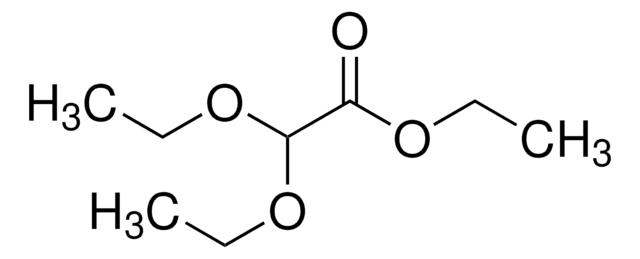
479047
2,2-Dimethoxyacetaldehyde solution
60 wt. % in H2O
Synonym(s):
Glyoxal 1-(dimethyl acetal), Glyoxal dimethyl acetal
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3O)2CHCHO
CAS Number:
Molecular Weight:
104.10
Beilstein:
1850744
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
concentration
60 wt. % in H2O
refractive index
n20/D 1.414
bp
100 °C
density
1.15 g/mL at 25 °C
functional group
acetal
aldehyde
ether
storage temp.
2-8°C
SMILES string
[H]C(=O)C(OC)OC
InChI
1S/C4H8O3/c1-6-4(3-5)7-2/h3-4H,1-2H3
InChI key
OGFKTAMJLKHRAZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
This product is a 60wt% solution of 2,2-dimethoxyacetaldehyde (glyoxal dimethyl acetal) in water. The aldol condensation of 2,2-dimethoxyacetaldehyde with acetoacetic ester in the absence of the catalyst and solvent has been reported. It participates in the synthesis of isoxazoline vinyl ester pseudopeptides and tetrahydro-β-carboline derivatives of barbituric acid analogs.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
169.0 °F
Flash Point(C)
76.1 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Catalyst-Free Aldol Additions of 1,3-Dicarbonyl Compounds.
Rohr K and Mahrwald R.
Advanced Synthesis & Catalysis, 350(18), 2877-2880 (2008)
Design and synthesis of Pictet-Spengler condensation products that exhibit oncogenic-RAS synthetic lethality and induce non-apoptotic cell death.
Skouta R, et al.
Bioorganic & Medicinal Chemistry Letters, 22(17), 5707-5713 (2012)
Synthesis and activity of isoxazoline vinyl ester pseudopeptides as proteasome inhibitors.
Marastoni M, et al.
Journal of Peptide Science, 20(4), 258-265 (2014)
Paolo Ziosi et al.
ChemSusChem, 11(13), 2202-2210 (2018-05-16)
A new process for the synthesis of hydroxytyrosol (3,4-dihydroxyphenylethanol), the most powerful natural antioxidant currently known, by means of a two-step approach is reported. Catechol is first reacted with 2,2-dimethoxyacetaldehyde in basic aqueous medium to produce the corresponding mandelic derivative
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service