190543
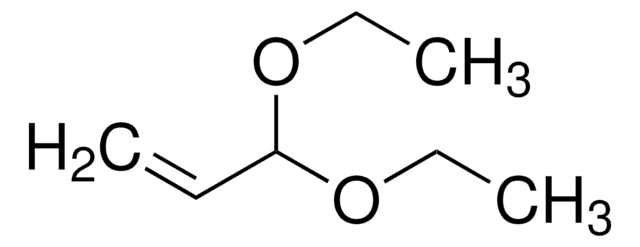
Acrolein dimethyl acetal
98%
Synonym(s):
3,3-Dimethoxy-1-propene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2C=CHCH(OCH3)2
CAS Number:
Molecular Weight:
102.13
Beilstein:
1700037
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.395 (lit.)
bp
89-90 °C (lit.)
density
0.862 g/mL at 25 °C (lit.)
functional group
acetal
ether
SMILES string
COC(OC)C=C
InChI
1S/C5H10O2/c1-4-5(6-2)7-3/h4-5H,1H2,2-3H3
InChI key
OBWGMYALGNDUNM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Acrolein dimethyl acetal undergoes cationic α-diimine palladium chelate catalyzed copolymerization with ethene to yield branched copolymers.
Application
Acrolein dimethyl acetal was used in the facile one step synthesis of 4-hydroxy-2E-nonenal and its dimethyl acetal via a cross-metathesis reaction with octen-3-ol.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
26.6 °F - closed cup
Flash Point(C)
-3 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Weidong Li et al.
Journal of the American Chemical Society, 126(39), 12246-12247 (2004-09-30)
Acrolein dimethyl acetal (ADMA) can be copolymerized with ethene using a cationic alpha-diimine palladium chelate catalyst to yield branched copolymers. Catalyst deactivation occurs via methanol elimination to give an inert eta3-1-methoxyallyl palladium species. This process can be retarded by the
Laurent Soulère et al.
Chemistry and physics of lipids, 150(2), 239-243 (2007-10-05)
The facile one step synthesis of 4-hydroxy-2E-nonenal and its dimethyl acetal via a cross-metathesis reaction between commercially available octen-3-ol and acrolein or its dimethyl acetal is reported. The method was extended to the synthesis of C6 and C12 4-hydroxy-2E-enals, their
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service