467448
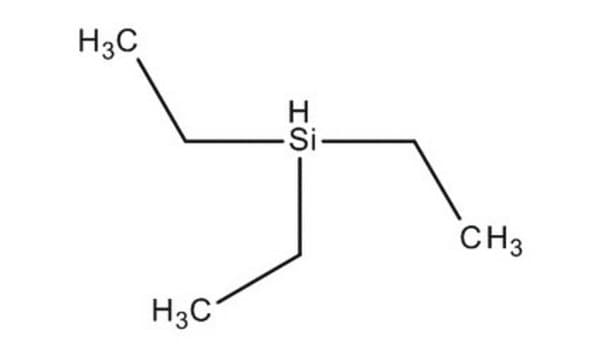
Triethylsilane
97%
Synonym(s):
NSC 93579, Triethylhydrosilane, Triethylsilicon hydride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C2H5)3SiH
CAS Number:
Molecular Weight:
116.28
Beilstein:
1098278
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
reaction suitability
reagent type: reductant
refractive index
n20/D 1.412 (lit.)
bp
107-108 °C (lit.)
density
0.728 g/mL at 25 °C (lit.)
SMILES string
CC[SiH](CC)CC
InChI
1S/C6H16Si/c1-4-7(5-2)6-3/h7H,4-6H2,1-3H3
InChI key
AQRLNPVMDITEJU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Catalyst for:
Catalyst reactivation after catalyst polymerization of styrene
Studies involving the prediction of organosilicon flash points
- Synthesis of a spiro-oxindole blocker of Nav1.7 for the treatment of pain
- Redox initiated cationic polymerization
- Beckmann rearrangement of cyclododecanone oxime
- Regioselective reductive coupling of enones and allenes
Catalyst reactivation after catalyst polymerization of styrene
Studies involving the prediction of organosilicon flash points
Triethylsilane can be used as:
- A reducing agent in the regioselective reductive coupling of enones and allenes.
- A reagent in the redox initiated cationic polymerization.
- A reagent in catalytic transfer hydrogenation, reduction of alkyl halides and silylation of aromatic C-H bonds, etc.
- As a reagent for the generation of indium hydride (Cl2InH) applicable as a catalyst for the intramolecular cyclization of enynes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
21.2 °F
Flash Point(C)
-6 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Iridium-catalyzed reduction of alkyl halides by triethylsilane.
Yang J and Brookhart M
Journal of the American Chemical Society, 129(42), 12656-12657 (2007)
Regioselective Nickel-Catalyzed Reductive Couplings of Enones and Allenes.
Li W, et al.
Angewandte Chemie (International Edition in English), 49(46), 8712-8716 (2010)
The ruthenium-catalyzed silylation of aromatic C-H bonds with triethylsilane.
Kakiuchi F, et al.
Journal of Organometallic Chemistry, 686(1-2), 134-144 (2003)
Redox initiated cationic polymerization.
Crivello JV
Journal of Polymer Science Part A: Polymer Chemistry, 47(7), 1825-1835 (2009)
Redox-Initiated Cationic Polymerization: Reduction of Dialkylphenacylsulfonium Salts by Silanes.
Molleo M and Crivello JV
Macromolecules, 42(12), 3982-3991 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service