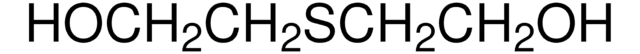380474
2-Hydroxyethyl disulfide
technical grade
Synonym(s):
2,2′-Dithiodiethanol, Bis(2-hydroxyethyl) disulfide
About This Item
Recommended Products
grade
technical grade
Quality Level
form
liquid
refractive index
n20/D 1.57 (lit.)
bp
158-163 °C/3.5 mmHg (lit.)
mp
25-27 °C (lit.)
density
1.261 g/mL at 25 °C (lit.)
SMILES string
OCCSSCCO
InChI
1S/C4H10O2S2/c5-1-3-7-8-4-2-6/h5-6H,1-4H2
InChI key
KYNFOMQIXZUKRK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- A chain extender in the preparation of waterborne self-healing polyurethane, which is used in leather coating.
- As a precursor in the synthesis of multifunctional initiator, which is used in the preparation of star polystyrenes by atom transfer radical polymerization of styrene.
- A starting material to prepare a disulfide-based branching agent. This branching agent is used to synthesize highly branched poly(2-hydroxypropyl methacrylate).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Over the past two decades, the rapid advance of controlled living polymerization (CLP) techniques.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service