264679
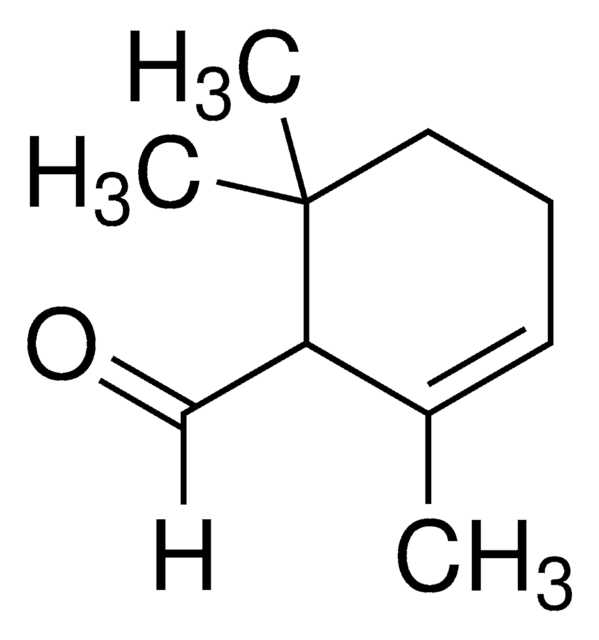
2,6,6-Trimethyl-1-cyclohexene-1-acetaldehyde
technical grade, 80%
Synonym(s):
β-Homocyclocitral
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H18O
CAS Number:
Molecular Weight:
166.26
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
80%
form
liquid
refractive index
n20/D 1.485 (lit.)
bp
58-59 °C/0.4 mmHg (lit.)
density
0.941 g/mL at 25 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)CC1=C(C)CCCC1(C)C
InChI
1S/C11H18O/c1-9-5-4-7-11(2,3)10(9)6-8-12/h8H,4-7H2,1-3H3
InChI key
VHTFHZGAMYUZEP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,6,6-Trimethyl-1-cyclohexene-1-acetaldehyde can be used as a starting material to prepare:
It can also be used as a key intermediate to synthesize drimane-related sesquiterpenes and substituted retinoic acid analogs.
- (±)-Aeginetolide by oxidation in the presence of meta-chloroperoxybenzoic acid (m-CPBA).
- (±)-Dihydroactinidiolide (a C11-terpenic lactone) via dehydration of key intermediate aeginetolide.
It can also be used as a key intermediate to synthesize drimane-related sesquiterpenes and substituted retinoic acid analogs.
Other Notes
Contains β-cyclocitral
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
An improved synthesis of (?)-dihydroactinidiolide.
Subbaraju GV, et al.
Tetrahedron Letters, 32(37), 4871-4874 (1991)
Improved Synthesis of 2, 6, 6-Trimethyl-1-cyclohexene-1-acetaldehyde, A Key Intermediate for Drimane-Related Sesquiterpenes.
de Jong JC, et al.
Synthetic Communications, 20(4), 589-596 (1990)
Improved Synthesis of 2, 6, 6-Trimethyl-1-cyclohexene-1-acetaldehyde, A Key Intermediate for Drimane-Related Sesquiterpenes
de Jong JC, et al.
Synthetic Communications, 20(4), 589-596 (1990)
An improved synthesis of (?)-dihydroactinidiolide
Subbaraju GV, et al.
Tetrahedron Letters, 32(37), 4871-4874 (1991)
Preparation and biological activity of 13-substituted retinoic acids
Wada A, et al.
Bioorganic & Medicinal Chemistry, 12(14), 3931-3942 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service