推荐产品
product name
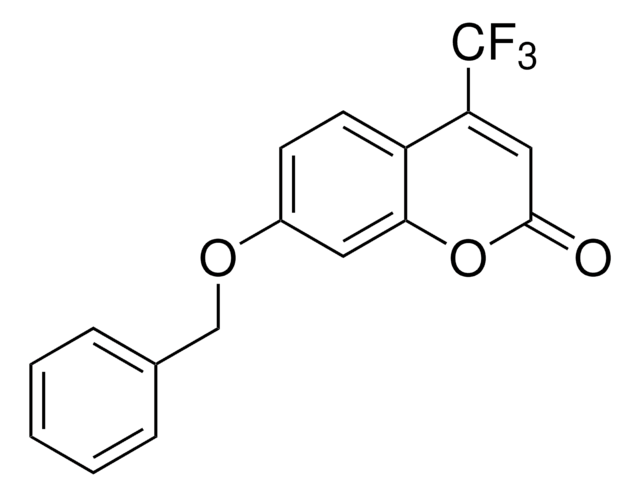
7-Ethoxy-4-(trifluoromethyl)coumarin, ≥98% (TLC)
品質等級
化驗
≥98% (TLC)
形狀
powder
溶解度
chloroform: 100 mg/mL, clear, colorless (Soluble in chloroform, methanol, and DMSO.)
螢光
λex 333 nm; λem 415 nm in methanol
儲存溫度
−20°C
SMILES 字串
CCOc1ccc2c(OC(=O)C=C2C(F)(F)F)c1
InChI
1S/C12H9F3O3/c1-2-17-7-3-4-8-9(12(13,14)15)6-11(16)18-10(8)5-7/h3-6H,2H2,1H3
InChI 密鑰
OLHOIERZAZMHGK-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
特點和優勢
7-Ethoxy-4-(trifluoromethyl)coumarin is a fluorogenic substrate for cytochrome P-450 catalyzed O-de-ethylation.
包裝
Bottomless glass bottle. Contents are inside inserted fused cone.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
J G DeLuca et al.
Biochemical pharmacology, 37(9), 1731-1739 (1988-05-01)
The microsomal O-deethylation of a novel coumarin analog, 7-ethoxy-4-trifluoromethylcoumarin (EFC), to a fluorescent product was characterized. Results indicate that this analog provides a rapid, convenient and highly sensitive means to assay cytochrome P-450-mediated metabolism. Like microsomal 7-ethoxycoumarin (7-EC) O-deethylation, EFC
Emily E Scott et al.
Chemical research in toxicology, 15(11), 1407-1413 (2002-11-20)
Until recently, all known structures of bacterial cytochromes P450 suggested that substrate access to the buried active site occurred via the F-G region, a surface loop distal to the heme cavity. However, the structure of P450 51 indicates a large
Chitra Sridar et al.
The Journal of pharmacology and experimental therapeutics, 301(3), 945-952 (2002-05-23)
Tamoxifen is primarily used in the treatment of breast cancer. It has been approved as a chemopreventive agent for individuals at high risk for this disease. Tamoxifen is metabolized to a number of different products by cytochrome P450 enzymes. The
Shih-Feng Lan et al.
Toxicology in vitro : an international journal published in association with BIBRA, 24(4), 1314-1323 (2010-02-23)
In this study, we have evaluated the use of ultra-sterile alginate hydrogels encapsulated with HepG2 liver cells for applications in high throughput drug screening. We have studied the cellular viability and metabolic capacity of the encapsulated cells in two different
Xilin Li et al.
Toxicological sciences : an official journal of the Society of Toxicology, 175(2), 251-265 (2020-03-12)
Metabolism plays a key role in chemical genotoxicity; however, most mammalian cells used for in vitro genotoxicity testing lack effective metabolizing enzymes. We recently developed a battery of TK6-derived cell lines that individually overexpress 1 of 8 cytochrome P450s (CYP1A1
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门