About This Item
推荐产品
品質等級
化驗
≥98% (TLC)
形狀
powder
儲存條件
(Tightly closed. Dry)
技術
activity assay: suitable
顏色
orange to red
mp
223-225 °C (lit.)
溶解度
chloroform: 9.80-10.20 mg/mL, clear, orange
適合性
suitable for fluorescence
儲存溫度
−20°C
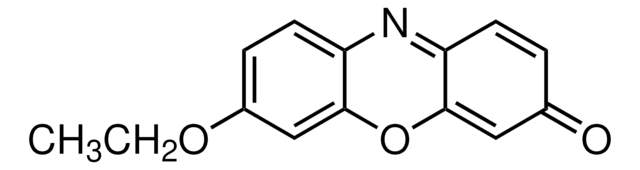
SMILES 字串
CCOc1ccc2N=C3C=CC(=O)C=C3Oc2c1.Fc4c(F)c(F)c(OC(=O)CNC(=O)OCC5c6ccccc6-c7ccccc57)c(F)c4F
InChI
1S/C23H14F5NO4.C14H11NO3/c24-17-18(25)20(27)22(21(28)19(17)26)33-16(30)9-29-23(31)32-10-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15;1-2-17-10-4-6-12-14(8-10)18-13-7-9(16)3-5-11(13)15-12/h1-8,15H,9-10H2,(H,29,31);3-8H,2H2,1H3
InChI 密鑰
ZOSYTBPPLWBBKM-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
适用研究领域:细胞信号传导
應用
基底
基底
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
商品
Phase I biotransformation reactions increase drug compound polarity, mainly occurring in hepatic circulation.
Phase I biotransformation reactions increase drug compound polarity, mainly occurring in hepatic circulation.
Phase I biotransformation reactions increase drug compound polarity, mainly occurring in hepatic circulation.
Phase I biotransformation reactions increase drug compound polarity, mainly occurring in hepatic circulation.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门