推荐产品
生物源
Streptomyces tubercidicus
品質等級
化驗
~95%
形狀
powder
顏色
off-white
抗生素活性譜
fungi
parasites
viruses
作用方式
DNA synthesis | interferes
SMILES 字串
O[C@H]1[C@@H](O)[C@H](N2C=CC3=C2N=CN=C3N)O[C@@H]1CO
InChI
1S/C11H14N4O4/c12-9-5-1-2-15(10(5)14-4-13-9)11-8(18)7(17)6(3-16)19-11/h1-2,4,6-8,11,16-18H,3H2,(H2,12,13,14)/t6-,7-,8-,11-/m1/s1
InChI 密鑰
HDZZVAMISRMYHH-KCGFPETGSA-N
基因資訊
rat ... Adora1(29290)
正在寻找类似产品? 访问 产品对比指南
一般說明
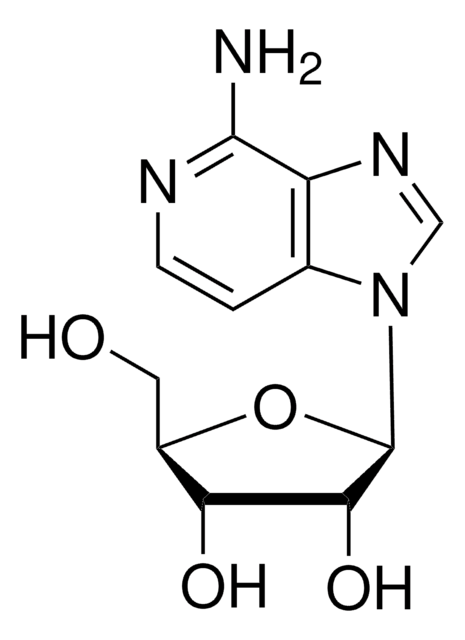
Chemical structure: nucleoside
應用
It is used to study tubercidin′s mechanism of toxicity, tubercidin resistance and is used as a selection agent. Tubercidin is used to study RNAi silencing. It is used to study the role of transmethylation in chemotaxis of eukaryotic cells.
生化/生理作用
Tubercidin is a toxic adenosine analog with antiviral, antitrypanosomal, and antifungal functions. It inhibits multiple metabolic processes, including RNA processing, nucleic acid synthesis, protein synthesis, and methylation of tRNA through intracellular incorporation into nucleic acids. Tubercidin acts as a plant antifungal, inhibits mammalian SAH hydrolase (SAHH), and blocks purine biosynthesis in Candida famata. Tubercidin inhibits glycolysis in Trypanosoma brucei. Tubercidin inhibits both chemotaxis and chemotaxis-dependent cell streaming of Dictyostelium, and chemotaxis of neutrophils at concentrations that have little effect on cell viability.
Toxic adenosine analog with antiviral, antitrypanosomal, and antifungal functions. Mode of action: Inhibits multiple metabolic processes, including RNA processing, nucleic acid synthesis, protein synthesis, and methylation of tRNA through intracellular incorporation into nucleic acids. Tubercidin acts as a plant antifungal, inhibits mammalian SAH hydrolase (SAHH), and blocks purine biosynthesis in Candida famata.
其他說明
Keep container tightly closed in a dry and well-ventilated place.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 2 Oral
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
其他客户在看
Aurelie Bourderioux et al.
Journal of medicinal chemistry, 54(15), 5498-5507 (2011-06-30)
A series of 7-aryl- and 7-hetaryl-7-deazaadenosines was prepared by the cross-coupling reactions of unprotected or protected 7-iodo-7-deazaadenosines with (het)arylboronic acids, stannanes, or zinc halides. Nucleosides bearing 5-membered heterocycles at the position 7 exerted potent in vitro antiproliferative effects against a
Misaki Matsui et al.
Oncogenesis, 9(6), 60-60 (2020-06-17)
The nucleus of mammalian cells is compartmentalized by nuclear bodies such as nuclear speckles, however, involvement of nuclear bodies, especially nuclear speckles, in DNA repair has not been actively investigated. Here, our focused screen for nuclear speckle factors involved in
Mark E Drew et al.
The Journal of biological chemistry, 278(47), 46596-46600 (2003-09-16)
We used an RNA interference (RNAi) library in a forward genetic selection to study the mechanism of toxicity of tubercidin (7-deazaadenosine) to procyclic Trypanosoma brucei. Following transfection of cells with an RNAi-based genomic library, we used 5 microm tubercidin to
Marco Singer et al.
Journal of the American Chemical Society, 132(24), 8372-8377 (2010-05-21)
Photochromic nucleosides were designed that combine the structural features and molecular recognition properties of nucleic acids with the light-sensitivity of diarylethenes. Target compounds 1a-c consist of a 7-deazaadenosine unit that is linked to a thiophene as the second aryl functionality
Ping Ding et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(48), 14385-14396 (2010-12-01)
Nucleobase-directed spin-labeling by the azide-alkyne 'click' (CuAAC) reaction has been performed for the first time with oligonucleotides. 7-Deaza-7-ethynyl-2'-deoxyadenosine (1) and 5-ethynyl-2'-deoxyuridine (2) were chosen to incorporate terminal triple bonds into DNA. Oligonucleotides containing 1 or 2 were synthesized on a
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门