推荐产品
生物源
human
品質等級
重組細胞
expressed in E. coli
化驗
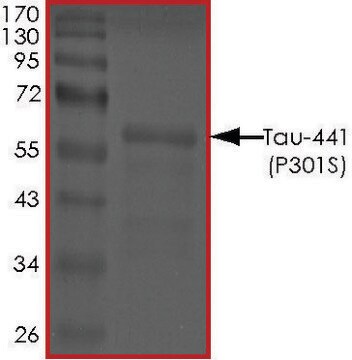
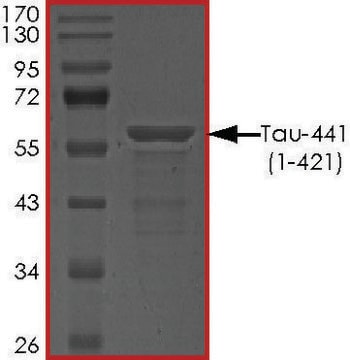
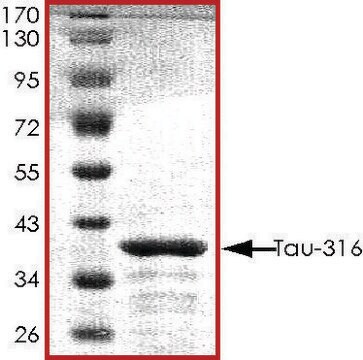
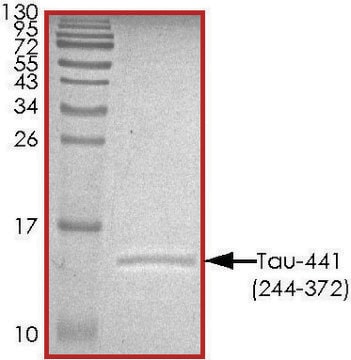
≥90% (SDS-PAGE)
形狀
lyophilized powder
分子量
42.9 kDa
UniProt登錄號
應用
cell analysis
運輸包裝
dry ice
儲存溫度
−70°C
基因資訊
human ... MAPT(4137)
一般說明
Tau-412 human, (1N4R) recombinant, is one of the isoforms of Tau and lacks a projection domain region. The isoforms of tau are generated by alternate splicing. The N terminal region of tau is acidic and is separated from the C-terminal tubulin-binding region by a central proline-rich region. The isoforms differ in amino-terminal N1 and N2 regions as well as binding repeats at the tubulin region. Tau belongs to the family of neuronal microtubule-associated proteins.
生化/生理作用
Tau promotes assembly and stabilizes neuronal microtubules under normal physiological conditions. Under pathological conditions Tau can also undergo modifications such as hyperphosphorylation, which can result in the generation of aberrant aggregates such as found in neurofibrillary tangles in Alzheimer′s disease. Tau-412 human is purified without any acid treatment and is suitable for promoting microtubule assembly, and for hyperphosphorylation-induced self-assembly into filaments.
Isoform of Tau, variant 1N4R, having 4 microtubule binding repeats (R) and one amino terminal insert (N).
重構
Lyophilized from MES, pH 6.8, containing NaCl and EGTA. When reconstituted in water to a protein concentration of 1 mg/mL, the resulting buffer will have ~50 mM MES, pH 6.8, 100 mM NaCl, and 0.5 mM EGTA.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Abolfazl Jangholi et al.
Archives of biochemistry and biophysics, 609, 1-19 (2016-09-18)
In many neurodegenerative diseases, formation of protein fibrillar aggregates has been observed as a major pathological change. Neurofibrillary tangles, mainly composed of fibrils formed by the microtubule-associated protein; Tau, are a hallmark of a group of neurodegenerative diseases such as
Simon Moussaud et al.
Molecular neurodegeneration, 9, 43-43 (2014-10-30)
The accumulation of α-synuclein aggregates is the hallmark of Parkinson's disease, and more generally of synucleinopathies. The accumulation of tau aggregates however is classically found in the brains of patients with dementia, and this type of neuropathological feature specifically defines
Jesus Avila et al.
Physiological reviews, 84(2), 361-384 (2004-03-27)
The morphology of a neuron is determined by its cytoskeletal scaffolding. Thus proteins that associate with the principal cytoskeletal components such as the microtubules have a strong influence on both the morphology and physiology of neurons. Tau is a microtubule-associated
A Himmler et al.
Molecular and cellular biology, 9(4), 1381-1388 (1989-04-01)
Tau proteins consist of a family of proteins, heterogeneous in size, which associate with microtubules in vivo and are induced during neurite outgrowth. In humans, tau is one of the major components of the pathognomonic neurofibrillary tangles in Alzheimer's disease
M Goedert et al.
Neuron, 3(4), 519-526 (1989-10-01)
We have determined the sequences of isoforms of human tau protein, which differ from previously reported forms by insertions of 29 or 58 amino acids in the amino-terminal region. Complementary DNA cloning shows that the insertions occur in combination with
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门