SML3256
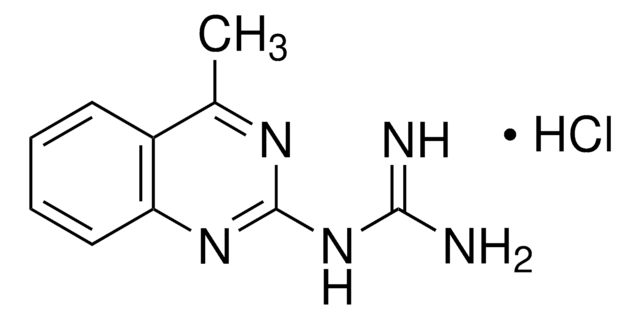
Fimasartan
≥98% (HPLC)
别名:
2-Butyl-1,6-dihydro-N,N,4-trimethyl-6-oxo-1-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-5-pyrimidineethanethioamide, 2-n-Butyl-5-dimethylaminothiocarbonylmethyl-6-methyl-3-[[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]pyrimidin-4(3H)-one, BR-A-657, BR-A657
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
品質等級
化驗
≥98% (HPLC)
形狀
powder
顏色
white to beige
溶解度
DMSO: 2 mg/mL, clear
儲存溫度
2-8°C
SMILES 字串
S=C(CC1=C(N=C(N(C1=O)CC2=CC=C(C=C2)C3=CC=CC=C3C4=NNN=N4)CCCC)C)N(C)C
InChI
1S/C27H31N7OS/c1-5-6-11-24-28-18(2)23(16-25(36)33(3)4)27(35)34(24)17-19-12-14-20(15-13-19)21-9-7-8-10-22(21)26-29-31-32-30-26/h7-10,12-15H,5-6,11,16-17H2,1-4H3,(H,29,30,31,32)
InChI 密鑰
AMEROGPZOLAFBN-UHFFFAOYSA-N
生化/生理作用
Fimasartan (BR-A-657) is an orally active, highly potent angiotensin II receptor AT1 (AGTR1) antagonist (blocker) that selectively blocks AngII-, but not KCl- or noradrenaline-, induced contraction of rabbit thoracic aortic rings (IC50 = 0.42 nM) with 615-fold higher AT1 affinity than losartan (IC50 = 0.13 nM vs. 80 nM against 0.05 nM AngII for binding rat adrenal cortex). Fimasartan significantly decreases mean arterial blood pressure with rapid onset (in 0.5 h) in spontaneously hypertensive rats (Emax of 75% reduction, 5-24 hr post 10 mg/kg p.o.) and is ~19-fold more effective than losartan against AngII (100 ng/kg i.v.)-induced pressor response in rats in vivo (ED50 = 18 ng/kg vs. 336 ng/kg i.v.).
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Jin Han et al.
International journal of cardiology, 168(3), 2851-2859 (2013-05-07)
The aim of this study was to investigate the cardioprotective effect of fimasartan, a newly developed angiotensin II receptor type I blocker (ARB), against myocardial ischemia/reperfusion (I/R) injury and to identify the mechanism by which it reduces mitochondrial damage. Fimasartan
Yong Ha Chi et al.
Biological & pharmaceutical bulletin, 36(7), 1208-1215 (2013-07-03)
The pharmacological profile of BR-A-657, 2-n-butyl-5-dimethylamino-thiocarbonyl-methyl-6-methyl-3-{[2-(1H-tetrazole-5-yl)biphenyl-4-yl]methyl}-pyrimidin-4(3H)-one, a new nonpeptide AT1-selective angiotensin receptor antagonist, has been investigated in a variety of in vitro and in vivo experimental models. In the present study, BR-A-657 displaced [(125)I][Sar(1)-Ile(8)]angiotensin II (Ang II) from its specific
Je Hak Kim et al.
Archives of pharmacal research, 35(7), 1123-1126 (2012-08-07)
Fimasartan (Kanarb®), an angiotensin II receptor antagonist with selectivity for the AT1 receptor subtype, is a pyrimidinone-related heterocyclic compound that was developed by Boryung Pharm. Co., Ltd. Among numerous synthetic derivatives, fimasartan was chosen as a new drug candidate through
Fabio Angeli et al.
Expert opinion on drug metabolism & toxicology, 14(5), 533-541 (2018-04-21)
Fimasartan is the ninth and latest Angiotensin Receptor Blockers for the treatment of hypertension. Fimasartan is a derivative of losartan in which the imidazole ring has been replaced. It provides a selective type 1 angiotensin II receptor antagonist effect with
Jung Won Kim et al.
Journal of clinical pharmacology, 53(1), 75-81 (2013-02-13)
The authors studied the effects of ketoconazole and rifampicin on the pharmacokinetics of a single dose of fimasartan (BR-A-657), a newly developed angiotensin II receptor antagonist for the treatment of hypertension, in 22 healthy participants. Ketoconazole increased the maximumplasma concentration
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门