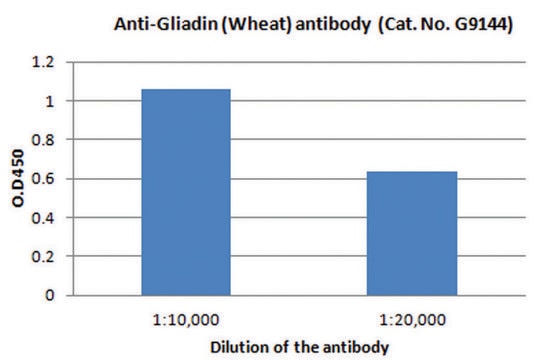
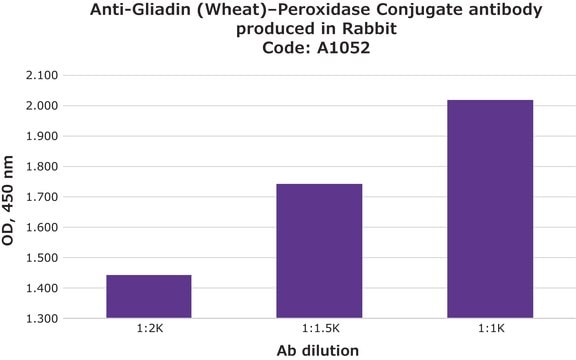
推荐产品
一般說明
Gliadin, a type of prolamin, the major component of wheat gluten, is comprised of single-chain polypeptides with an average molecular weight of 25–100 kDa linked by intramolecular disulfide bonds.1 The term gliadin defines a group of proteins extracted from gluten by 70% ethanol. All fractions have remarkably low solubility in aqueous solution except at extreme pHs2. This low water solubility has been attributed to the presence of disulfide bonds together with cooperative hydrophobic interactions within the protein folding and structure,. The amino acid composition shows that gliadin has equal amounts of polar and neutral amino acids, mainly glutamine (about 40%) in addition to high proline content (14%).
As plant proteins, they are recognized as prion-free 2.Gliadin can adhere to the mucus layer of the stomach due to its mucoadhesive property, which is a result of various interactions (e.g., hydrogen bonding, van der Waals force, and mechanical penetration).
As plant proteins, they are recognized as prion-free 2.Gliadin can adhere to the mucus layer of the stomach due to its mucoadhesive property, which is a result of various interactions (e.g., hydrogen bonding, van der Waals force, and mechanical penetration).
特異性
Monoclonal Anti-Gliadin antibody specifically recognizes wheat gliadin. The antibody is specific for wheat, barley, spelt, rye. No cross reaction is observed with oats, soya, corn, rice and potato extracts.
應用
The antibody may be used in various immunochemical techniques including Immunoblotting and ELISA.
生化/生理作用
Gliadins, from an alcohol-soluble fraction of gluten, are storage proteins in wheat, barley, and rye (along with other gluten-containing grains). Glutenins are insoluble in alcohol and differ in their biochemical structure from glutens. It has been hypothesized that the initial immune reaction in celiac disease patients is focused on several of gliadin peptides, while the long-standing inflammatory response may be defined by other gliadin peptides that have been deamidated or cross-linked by tissue transglutaminase. These latter peptides are also more tightly linked to HLA-DQ2 and HLA-DQ83.
The tissue transglutaminase-catalyzed changes in gliadin are not restricted to single gliadin types, for example, prolamines in barley and rye (known as hordelin and secalin, respectively) and these may induce a messenger RNA-mediated interferon-gamma response in the small-intestinal mucosa of celiacs. An alpha-2-gliadin-33-mer appears to pass through the enterocyte brush border membrane by a dose-dependent translocation mechanism, and this is further enhanced by interferon-gamma, possibly mediated by a delayed epidermal growth factor endocytosis. In active celiac disease, this immune-mediated response is very complex and involves many different mediators (such as Interlukin-21 from activated CD4+ Tcells) some are still being studied.
With genetic susceptibility, gliadin may interact with interenterocyte tight junctions and cause their disassembly. Moreover, Gliadin peptides may bind to the chemokine receptor, CXCR3. This induces zonulin release from the tight junction region and causes a resultant increase in intestinal permeability. Permeability increases have also been demonstrated prior to the onset of clinically apparent celiac disease, and even with a gluten-free diet, this altered tight junction permeability may not completely return to normal3.Anti Gliadin antibody may serve as an important tool in Celiac disease research field.
The tissue transglutaminase-catalyzed changes in gliadin are not restricted to single gliadin types, for example, prolamines in barley and rye (known as hordelin and secalin, respectively) and these may induce a messenger RNA-mediated interferon-gamma response in the small-intestinal mucosa of celiacs. An alpha-2-gliadin-33-mer appears to pass through the enterocyte brush border membrane by a dose-dependent translocation mechanism, and this is further enhanced by interferon-gamma, possibly mediated by a delayed epidermal growth factor endocytosis. In active celiac disease, this immune-mediated response is very complex and involves many different mediators (such as Interlukin-21 from activated CD4+ Tcells) some are still being studied.
With genetic susceptibility, gliadin may interact with interenterocyte tight junctions and cause their disassembly. Moreover, Gliadin peptides may bind to the chemokine receptor, CXCR3. This induces zonulin release from the tight junction region and causes a resultant increase in intestinal permeability. Permeability increases have also been demonstrated prior to the onset of clinically apparent celiac disease, and even with a gluten-free diet, this altered tight junction permeability may not completely return to normal3.Anti Gliadin antibody may serve as an important tool in Celiac disease research field.
外觀
Supplied as a solution in 0.01 M phosphate buffered saline pH 7.4, containing 15 mM sodium azide as a preservative.
儲存和穩定性
For continuous use, store at 2-8°C for up to one month. For extended storage, freeze in working aliquots. Repeated freezing and thawing is not recommended. If slight turbidity occurs upon prolonged storage, clarify the solution by centrifugation before use. Working dilution samples should be discarded if not used within 12 hours.
免責聲明
Unless otherwise stated in our catalog our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
儲存類別代碼
12 - Non Combustible Liquids
水污染物質分類(WGK)
nwg
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Naiyana Gujral et al.
World journal of gastroenterology, 18(42), 6036-6059 (2012-11-17)
Celiac disease (CD) is one of the most common diseases, resulting from both environmental (gluten) and genetic factors [human leukocyte antigen (HLA) and non-HLA genes]. The prevalence of CD has been estimated to approximate 0.5%-1% in different parts of the
Ahmed O Elzoghby et al.
Journal of controlled release : official journal of the Controlled Release Society, 161(1), 38-49 (2012-05-09)
Among the available potential colloidal drug carrier systems, protein-based nanocarriers are particularly interesting. Meeting requirements such as low cytotoxicity, abundant renewable sources, high drug binding capacity and significant uptake into the targeted cells, protein-based nanocarriers represent promising candidates for efficient
Implications of protein- and Peptide-based nanoparticles as potential vehicles for anticancer drugs.
Ahmed O Elzoghby et al.
Advances in protein chemistry and structural biology, 98, 169-221 (2015-03-31)
Protein-based nanocarriers have gained considerable attention as colloidal carrier systems for the delivery of anticancer drugs. Protein nanocarriers possess various advantages including their low cytotoxicity, abundant renewable sources, high drug-binding capacity, and significant uptake into the targeted tumor cells. Moreover
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门