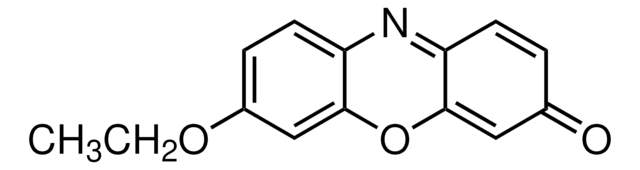
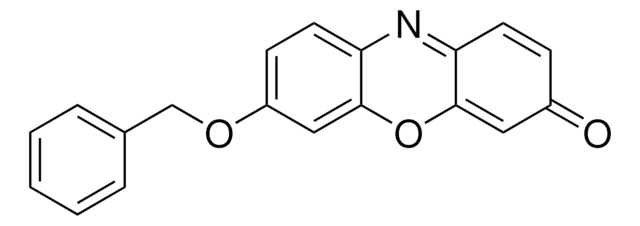
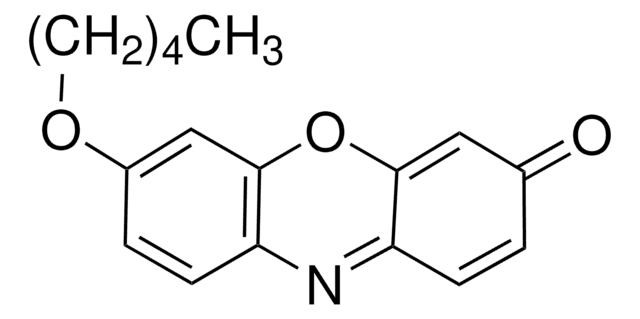
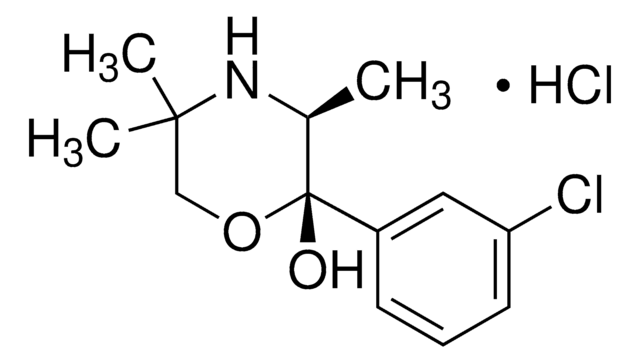
推荐产品
應用
Resorufin methyl ether have been used as substrate in the incubation mixture, during the determination of cytochrome P4501A activities such as ethoxyresorufin O-deethylase (EROD) and methoxyresorufin O-demethylase(MROD) in liver microsomes, using high performance liquid chromatography(HPLC).
生化/生理作用
Fluorimetric substrate for cytochrome P450 linked enzymes.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Dissecting the insecticide-resistance- associated cytochrome P450 gene Cyp6g1.
McCart C and ffrench-Constant R H
Pest Management Science, 64(6), 639-645 (2008)
Determination of cytochrome P450 1A activities in mammalian liver microsomes by high-performance liquid chromatography with fluorescence detection
Hanioka N, et al.
Journal of Chromatography. B, Biomedical Sciences and Applications, 744(2), 399-406 (2000)
Caroline McCart et al.
Pest management science, 64(6), 639-645 (2008-03-14)
The cytochrome P450 gene Cyp6g1 is overtranscribed in all field isolates of DDT-resistant Drosophila melanogaster (Meigen) and confers a fitness advantage when inherited via the female. Overtranscription is associated with the insertion of an Accord transposable element into the 5'
Xiaowei Zhang et al.
Toxicology and applied pharmacology, 234(3), 306-313 (2008-12-02)
As part of an ongoing effort to understand aryl hydrocarbon receptor (AhR) mediated toxicity in mink, cDNAs encoding for CYP1A1 and the CYP1A2 mixed function monooxygenases were cloned and characterized. In addition, the effects of selected dibenzofurans on the expression
Nicholas E Hadjokas et al.
British journal of pharmacology, 136(3), 347-352 (2002-05-25)
1. Cytochrome P4501A2 (CYP1A2) activates a large number of procarcinogens to carcinogens. Phytochemicals such as flavones can inhibit CYP1A2 activity competitively, and hydroxylated derivatives of flavone (galangin) may be potent, selective inhibitors of CYP1A2 activity relative to CYP1A1 activity. Molecular
商品
Phase I biotransformation reactions increase drug compound polarity, mainly occurring in hepatic circulation.
Phase I biotransformation reactions increase drug compound polarity, mainly occurring in hepatic circulation.
Phase I biotransformation reactions increase drug compound polarity, mainly occurring in hepatic circulation.
Phase I biotransformation reactions increase drug compound polarity, mainly occurring in hepatic circulation.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门