About This Item
推荐产品
生物源
synthetic
化驗
≥90% (HPLC)
形狀
powder
儲存條件
protect from light
顏色
yellow
mp
277 °C
溶解度
ethanol: 20 mg/mL
DMSO: 50 mg/mL
儲存溫度
room temp
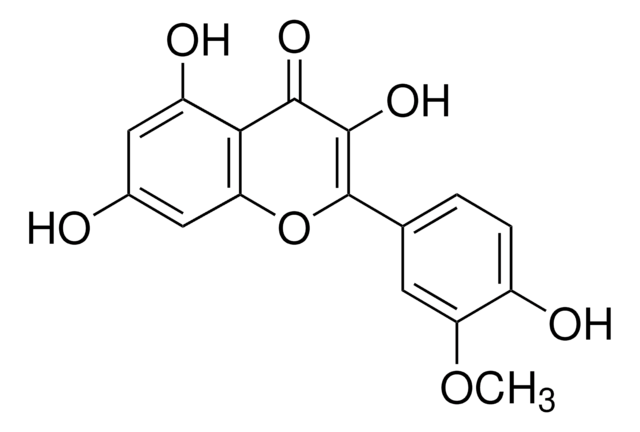
SMILES 字串
Oc1ccc(cc1)C2=C(O)C(=O)c3c(O)cc(O)cc3O2
InChI
1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H
InChI 密鑰
IYRMWMYZSQPJKC-UHFFFAOYSA-N
基因資訊
human ... CDC2(983) , CDK5(1020) , CDK6(1021) , CYP1A2(1544) , CYP2C9(1559) , GSK3A(2931)
mouse ... Hexa(15211)
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
- 检测其作为抗氧化剂和神经保护剂,对SH-S5Y5细胞鱼藤酮诱导的帕金森病模型的潜在影响
- 检测其对人主动脉内皮细胞(HAEC)脂多糖(LPS)诱导炎性损伤的抗炎作用
- 研究其通过抑制核因子E2相关因子(Nrf2)而对非小细胞肺癌细胞产生的细胞凋亡敏化作用
生化/生理作用
包裝
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
其他客户在看
商品
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
Fatty acid synthesis supports cancer cell proliferation, essential for membrane generation, protein modification, and bioenergetics.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门