所有图片(1)
About This Item
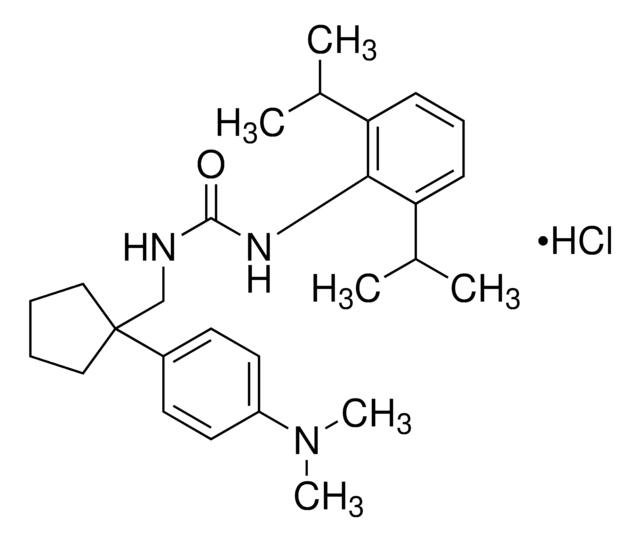
经验公式(希尔记法):
C23H39NO4
CAS号:
分子量:
393.56
MDL编号:
UNSPSC代码:
51111800
PubChem化学物质编号:
NACRES:
NA.77
推荐产品
质量水平
方案
>98% (HPLC)
表单
solid
溶解性
DMSO: >10 mg/mL
H2O: insoluble <2 mg/mL
储存温度
2-8°C
SMILES字符串
CCCCCCCCCCC(C)(C)C(=O)Nc1c(OC)cc(OC)cc1OC
InChI
1S/C23H39NO4/c1-7-8-9-10-11-12-13-14-15-23(2,3)22(25)24-21-19(27-5)16-18(26-4)17-20(21)28-6/h16-17H,7-15H2,1-6H3,(H,24,25)
InChI key
WAFNZAURAWBNDZ-UHFFFAOYSA-N
应用
CI 976 has been used as an acyl-coenzyme A: cholesterol acyltransferase (ACAT) inhibitor:
- to analyze its anti-hepatitis C virus (HCV) activity in Huh7.5.1 cells
- to treat Neuro-2a cells to test its effect on plasma membrane integrated density of α4-SEPβ2 or α6-SEPβ2β3 nicotinic acetylcholine receptors (nAChRs)
- to study its effects on the anti-angiogenic activity of pyripyropenes in human umbilical vein endothelial cells
生化/生理作用
CI-976 is a potent and specific inhibitor of liver and intestinal acyl coenzyme A:cholesterol acyltransferase (ACAT) in vitro.
CI-976, a new trimethoxy fatty acid anilide, is a potent and specific inhibitor of liver and intestinal acyl coenzyme A; cholesterol acyltransferase (ACAT) in vitro. CI-976 decreased non-high density lipoprotein (HDL)-cholesterol and increased HDL-cholesterol in rats with pre-established dyslipidemia. High performance gel chromatographic separation of plasma lipoproteins also revealed that CI-976, but not CL 277,082, lowered low density lipoprotein (LDL)-cholesterol and elevated HDL-cholesterol.
危险声明
预防措施声明
危险分类
Aquatic Chronic 4
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type N95 (US)
Y Matsui et al.
Japanese journal of pharmacology, 85(4), 423-433 (2001-06-05)
R-755 (N-(2,6-diethylphenyl)-N'-[3-(2-methylphenyl)-6,7-dihydro-5H-cyclopenta[f[l]benzothiophen-2-yl]urea), a novel acyl-CoA:cholesterol acyltransferase (ACAT) inhibitor, has been characterized in vitro, ex vivo and in vivo. R-755 potently inhibited ACAT activities, with IC50 values from 2.5 to 64 nM, in rabbit intestinal microsomes and several cell lines (CaCo-2
Daniel Drecktrah et al.
Molecular biology of the cell, 14(8), 3459-3469 (2003-08-20)
Recent studies have suggested that formation of Golgi membrane tubules involves the generation of membrane-associated lysophospholipids by a cytoplasmic Ca2+-independent phospholipase A2 (PLA2). Herein, we provide additional support for this idea by showing that inhibition of lysophospholipid reacylation by a
Asami Hayashi et al.
Biological & pharmaceutical bulletin, 32(7), 1261-1265 (2009-07-03)
In the course of our search for anti-angiogenic substances, pyripyropenes A (1), B (2), and D (3) were re-discovered as selective anti-proliferative substances against human umbilical vein endothelial cells (HUVECs) from a marine-derived fungus of Aspergillus sp. Pyripyropenes showed potent
S Perrey et al.
Atherosclerosis, 155(2), 359-370 (2001-03-20)
The cholesteryl ester, foam cell-enriched vulnerable plaque is a principle pharmacological target for reducing athero-thrombosis. Acyl CoA:cholesterol Acyl Transferase (ACAT) catalyzes the esterification of free cholesterol in intestine, liver, adrenal and macrophages, leading in the latter cells to intracellular cholesteryl
R A Harte et al.
Biochimica et biophysica acta, 1258(3), 241-250 (1995-10-05)
The effect of the membrane environment of acyl-CoA:cholesterol acyl transferase (ACAT), an important intracellular enzyme of cholesterol metabolism, on the properties of a range of inhibitors of varying potencies was studied. ACAT activity from rat liver was solubilised with 3%
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门