所有图片(1)
About This Item
经验公式(希尔记法):
C21H24N2O3
CAS号:
分子量:
352.43
Beilstein:
97268
EC 号:
MDL编号:
UNSPSC代码:
12352200
NACRES:
NA.25
推荐产品
方案
≥98.0% (HPLC)
旋光性
[α]/D -65±3°, c = 1 in chloroform
mp
~258 °C (dec.)
应用
metabolomics
vitamins, nutraceuticals, and natural products
储存温度
2-8°C
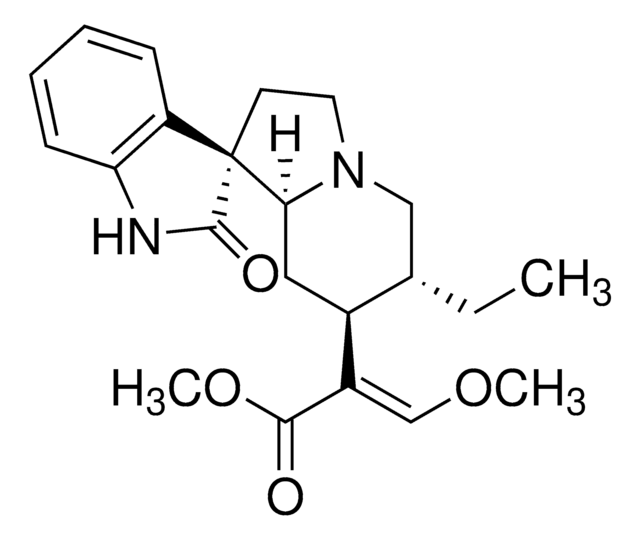
SMILES字符串
N21[C@@H](C[C@H]5[C@@H]([C@@H](OC=C5C(=O)OC)C)C2)c3[nH]c4c(c3CC1)cccc4
InChI
1S/C21H24N2O3/c1-12-16-10-23-8-7-14-13-5-3-4-6-18(13)22-20(14)19(23)9-15(16)17(11-26-12)21(24)25-2/h3-6,11-12,15-16,19,22H,7-10H2,1-2H3/t12-,15-,16+,19-/m0/s1
InChI key
GRTOGORTSDXSFK-XJTZBENFSA-N
正在寻找类似产品? 访问 产品对比指南
应用
Ajmalicine (δ-Yohimbine, Py-Tetrahydroserpentine, Raubasine) is an alkaloid used to study its effects as an antagonist of adrenergic and nicotinic receptors.
生化/生理作用
Metabolite in the indole alkaloid biosynthesis (serpentine production); found naturally in various plants such as Rauwolfia spp., Catharanthus roseus, and Mitragyna speciosa. It shows antimicrobial activity, and is used as an anti-hypertensive and sedative.
包装
Bottomless glass bottle. Contents are inside inserted fused cone.
警示用语:
Danger
危险声明
危险分类
Acute Tox. 2 Oral
储存分类代码
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
Catharanthus alkaloids and their enhanced production using elicitors: a review.
Gautam, S., et al.
International Journal of Pharmacy and Technology, 3, 713-724 (2011)
Trends for diverse production strategies of plant medicinal alkaloids.
Liuqing Yang et al.
Natural product reports, 27(10), 1469-1479 (2010-08-24)
N M Rojas Hernández
Revista cubana de medicina tropical, 31(3), 199-204 (1979-09-01)
In pursuing the study of the antimicrobial properties of alkaloids prepared from Cuban plants the activity of 10 indol alkaloids and 4 semisynthetic variables obtained from three plants--Catharanthus roseus G. Don., Vallesia antillana Wood and Ervatamia coronaria Staph, of the
J Roquebert
Archives internationales de pharmacodynamie et de therapie, 282(2), 252-261 (1986-08-01)
The selectivity of raubasine and its two isomers tetrahydroalstonine (THA) and akuammigine (AKU) for alpha 1- and alpha 2-adrenoceptors has been investigated in pithed normotensive rats. alpha 1-Adrenoceptor blockade was measured by inhibition of the pressor response to (-)-phenylephrine. alpha
Roberts, M. F.
Alkaloids: biochemistry, ecology, and medicinal applications, 450-450 (1998)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门