推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to Ph. Eur. S1800000
traceable to USP 1625009
API 家族
sulfadiazine
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
mp
253 °C (dec.) (lit.)
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-30°C
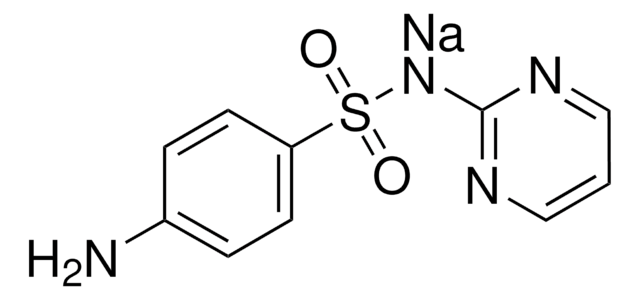
SMILES 字串
Nc1ccc(cc1)S(=O)(=O)Nc2ncccn2
InChI
1S/C10H10N4O2S/c11-8-2-4-9(5-3-8)17(15,16)14-10-12-6-1-7-13-10/h1-7H,11H2,(H,12,13,14)
InChI 密鑰
SEEPANYCNGTZFQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
Sulfadiazine is a bacteriostatic drug, that belongs to the class of medication referred as sulfonamide antibiotics. It is generally used in the treatment of cerebral meningitis, pneumonia, staphylococcal and streptococcal sepsis.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
應用
Sulfadiazine may be used as a pharmaceutical reference standard for the determination of the analyte in bulk drug and pharmaceutical formulations by high performance liquid chromatography.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析報告
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAC3953 in the slot below. This is an example certificate only and may not be the lot that you receive.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 2 - Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
其他客户在看
Sulfadiazine
European Pharmacopoeia Commission and European Directorate for the Quality of Medicines & Healthcare
European pharmacopoeia, 3674-3675 (2017)
Simultaneous determination of enrofloxacin, silver sulfadiazine, hydrocortisone acetate, hydrocortisone sodium succinate, and preservative excipients in pharmaceutical preparations using HPLC-DAD method
Rosa AM, et al.
Chromatographia, 80(11), 1641-1649 (2017)
Sulfonamide antibiotics
Connor EE
Primary Care Update for OB/GYNS, 5(1), 32-35 (1998)
Sulfadiazine
Pharmacopeia, US
United States Pharmacopeia/National Formulary, 4106-4106 (2018)
Determination of the content and the related substances of silver sulfadiazine gel by HPLC
Li Y, et al.
Chinese Journal of Hospital Pharmacy, 24(8), 474-474 (2004)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门