推荐产品
等級
pharmaceutical primary standard
API 家族
nimodipine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
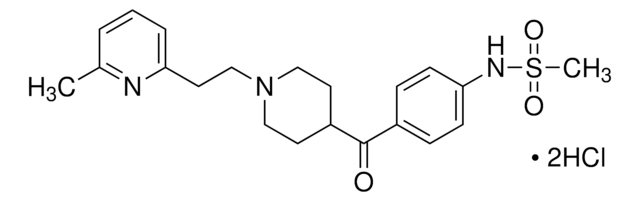
SMILES 字串
COCCOC(=O)C1=C(C)NC(C)=C(C1c2cccc(c2)[N+]([O-])=O)C(=O)OC(C)C
InChI
1S/C21H26N2O7/c1-12(2)30-21(25)18-14(4)22-13(3)17(20(24)29-10-9-28-5)19(18)15-7-6-8-16(11-15)23(26)27/h6-8,11-12,19,22H,9-10H2,1-5H3
InChI 密鑰
UIAGMCDKSXEBJQ-UHFFFAOYSA-N
基因資訊
human ... CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779) , NR3C2(4306)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Nimodipine EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
J Horn et al.
Stroke, 32(10), 2433-2438 (2001-10-06)
Based on the results of animal experiments, clinical trials were performed with nimodipine, which did not demonstrate a beneficial effect on outcome after stroke. The aim of this study was to determine whether the evidence from animal experiments with nimodipine
W Mück et al.
Die Pharmazie, 49(2-3), 130-139 (1994-02-01)
The analytical test procedures currently established for the determination of the dihydropyridine calcium antagonist nimodipine in biological fluids are presented. Method of choice which has been dominantly used in pharmacokinetic investigations and drug interaction studies is gas chromatography with electron-capture
Potential interactions between nimodipine and adrenal hormones.
R L Isaacson et al.
Annals of the New York Academy of Sciences, 765, 134-142 (1995-09-15)
Mervyn D I Vergouwen et al.
The Lancet. Neurology, 5(12), 1029-1032 (2006-11-18)
Despite several randomised controlled trials, there is still much debate whether nimodipine improves outcome in patients with traumatic subarachnoid haemorrhage. A 2003 Cochrane review reported improved outcome with nimodipine in these patients; however, because the results of Head Injury Trial
Daniele Tomassoni et al.
Clinical and experimental hypertension (New York, N.Y. : 1993), 30(8), 744-766 (2008-11-21)
Nimodipine is a 1,4-dihydropyridine-derivative Ca(2+)-channel blocker developed approximately 30 years ago. It is highly lipophilic, crosses the blood-brain barrier, and reaches brain and cerebrospinal fluid. Early treatment with nimodipine reduces the severity of neurological deficits resulting from vasospasm in subarachnoid
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门